The management of congenital heart disease (CHD) has evolved, improving patient outcomes; however, challenges persist for patients, emphasizing the importance of assessing health-related quality of life (HRQoL). The widely used Pediatric Quality of Life Inventory underscores the relevance of HRQoL assessment, especially in children subject to medical procedures.
ObjectiveTo evaluate HRQoL in children with congenital heart disease undergoing cardiac catheterization, analysing its association with clinical and sociodemographic variables in a tertiary care hospital.
Materials and methodsWe conducted a cross-sectional study in paediatric patients aged 2–18 years undergoing haemodynamic procedures for congenital heart diseases. We used the Pediatric Quality of Life Inventory (PedsQL) to assess HRQoL. The statistical analysis included descriptive statistics, χ2 tests, Kruskal–Wallis tests and multivariate linear regression analysis with the aim of identifying factors associated with HRQoL.
ResultsThe sample included 164 patients, among whom pulmonary atresia and patent ductus arteriosus were frequent diagnoses. Physical functioning and school functioning were significantly impaired, with median scores of 32.14 (IQR, 17.14–62.87) and 56 (IQR, 28–88), respectively. The results were more favourable for emotional functioning and social functioning, with median scores of 62 (IQR, 32–74) and 68 (IQR, 44–100), respectively. Single ventricle defects and pulmonary atresia were associated with lower quality of life scores in emotional functioning (P = .035) and physical functioning (P = .048), respectively.
ConclusionThis study highlights the current challenges in evaluating HRQoL for children with CHD. It identified significant associations between specific diagnoses and decreased HRQoL scores, emphasizing the need for comprehensive care strategies.
El manejo de la cardiopatía congénita (CC) ha evolucionado, mejorando el pronóstico, pero persisten desafíos para los pacientes, subrayando la importancia de evaluar la calidad de vida relacionada con la salud (CVRS). El cuestionario de calidad de vida pediátrica PedsQL, ampliamente utilizado, resalta la relevancia de la evaluación de CVRS, especialmente en niños sometidos a intervenciones médicas.
ObjetivoEvaluar la CVRS de niños con cardiopatía congénita sometidos a cateterismo cardíaco, examinando su relación con variables clínicas y sociodemográficas en una institución de alta complejidad.
Materiales y métodosSe llevó a cabo un estudio transversal en pacientes pediátricos de 2 a 18 años sometidos a cateterismo para patologías congénitas. Se utilizó el cuestionario de calidad de vida pediátrica PedsQL para evaluar la CVRS. El análisis estadístico incluyó estadísticas descriptivas, pruebas de chi-cuadrado, pruebas de Kruskal-Wallis y modelos de regresión lineal multivariante con el objetivo de identificar factores asociados.
ResultadosEl estudio involucró a 164 pacientes, y la atresia pulmonar y ductus arterioso siendo diagnósticos comunes. La actividad física y la escolarización se vieron significativamente afectadas, con puntuaciones medianas de 32,14 (IR 17,14–62,87) y 56 (IR 28 - 88), respectivamente. El estado emocional y la actividad social mostraron mejores resultados, con puntuaciones medianas de 62 (IR 32–74) y 68 (IR 44–100), respectivamente. El diagnóstico de ventrículo único y la atresia pulmonar se asociaron con puntuaciones más bajas de calidad de vida en el estado emocional (p = 0,035) y la función física (p = 0.048), respectivamente.
ConclusiónEste estudio subraya los desafíos continuos en la evaluación de la CVRS para niños con CC. Destaca asociaciones significativas entre diagnósticos específicos y puntuaciones disminuidas de CVRS, enfatizando la necesidad de estrategias de atención integral.
Congenital heart disease (CHD) is a term encompassing significant structural abnormalities of the heart or thoracic great vessels present at birth that may have functional implications.1 It is the most prevalent type of congenital disease and its incidence is of approximately 10–12 cases per 1000 live births.2 It is also the leading cause of infant mortality among the birth defects.3 However, the landscape of CHD management has evolved drastically. Advancements in cardiovascular knowledge and the relentless pursuit of novel diagnostic, interventional, and surgical approaches have revolutionized patient care.4 Still, the severity of congenital heart disease dictates its prognosis, and affected patients face complications of varying degree.5
Variations in the severity of CHD, coupled with inconsistencies in its definition across studies, give rise to significant heterogeneity in the estimates of its incidence, ranging from 4 to 50 cases per 1000 live births.6 Despite these challenges, the prognosis for infants diagnosed with CHD has markedly improved. Currently, 96% of newborns who survive their first year with CHD are expected to reach the age of 16.7 The median age of patients with severe CHD has increased from 11 years in 1985 to 17 years in 2000, and the overall median age at death has increased from 37 years in 2002 to 57 years in 2007.8 This positive trend is also marked by a substantial decline in mortality among patients with CHD over the past two decades, with reductions ranging between 50% and 70% depending on the specific defect. These findings evince substantial progress in the management and treatment of CHD, reflecting the effectiveness of advancements in medical care and interventions.9
Cardiac catheterization has emerged as a fundamental tool in the diagnosis and treatment of patients with CHD. This technique allows a thorough evaluation of cardiac anatomy and function, facilitating the detection of anomalies and providing precise information to guide therapeutic interventions.10 It plays a crucial role in the management of cardiac diseases in paediatric patients by enabling minimally invasive therapeutic procedures, which can improve their quality of life and long-term outcomes. It is particularly useful in patients with more complex conditions in whom thorough evaluation and management are required.11
Despite efforts to address the needs of these patients, they may still encounter various barriers in their daily lives, including difficulties in regular schooling, mobility restrictions, limitations in daily activities, changes in interpersonal relationships with both parents and peers, and challenges adapting to their circumstances including physical, psychosocial, cognitive and emotional issues.12 Apart from the inherent challenges posed by their underlying medical condition, these patients face additional complexities due to the impact on their quality of life across social, educational, and emotional domains. In this context, integrating the assessment of quality of life emerges as a crucial component of the medical care provided to these patients.13 Over the past few decades, significant progress has been made in the management of CHD, with nearly 95% of diagnosed children successfully transitioning into adolescence and adulthood. However, adolescents with CHD experience a decline in their health-related quality of life (HRQoL).14
Health-related quality of life is a multidimensional and subjective construct that encompasses various aspects of wellbeing, including physical, social, and psychological functioning. In the paediatric context, the assessment of HRQoL emerges as an imperative need, extending beyond the clinical domain to provide a comprehensive view of the wellbeing of children and adolescents from the perspective of either the patient or the parents.15 Several instruments have been developed for the purpose, including the Child Health Questionnaire (CHQ),16 the KIDSCREEN questionnaire,17 the Youth Quality of Life Instrument (YQOL),18 the Child Health and Illness Profile (CHIP)19 and the Pediatric Quality of Life Inventory (PedsQL).20 Among these, the PedsQL stands out for its widespread use and cross-cultural validity, is available in multiple languages and is frequently employed in paediatric health research.20
The PedsQL, developed by Professor Varni and his team in the United States in 1987, is a fundamental tool for assessing the quality of life of children aged 2–18 years.21 Its generic core scale (PedsQL 4.0) includes 23 items distributed in four dimensions: physical functioning, emotional functioning, social functioning and school functioning.20 Within this framework, the main aim of our study was to assess the HRQoL of children with CHD who underwent cardiac catheterization at a single tertiary care hospital, and analyse its association with clinical and sociodemographic factors.
Material and methodsWe conducted a cross-sectional analytical study in patients aged 2–18 years with CHD undergoing paediatric hemodynamic procedures in 2023 at a tertiary care hospital that is a reference centre for cardiovascular disease. We collected data from databases developed in the REDCap platform as part of the institutional follow-up project PID 366 and the department of paediatric haemodynamics project PID 520.22,23 The institutional project records data on the quality of life during the patient’s hospital stay collected by nursing staff through face-to-face or telephone interviews. The haemodynamics project data are collected through direct entry by by the paediatric haemodynamic specialist performing the procedure and the automated capture of data from the electronic health record database of the hospital. We excluded patients with lengths of stay of less than 24 h and international patients.
The variables under the study included sociodemographic factors such as sex and age and anthropometric measurements such as weight, height, and body surface area. We also collected data for clinical variables including the primary diagnosis, time of diagnosis (at birth or postoperative), level of risk assessed with the Catheterization for Congenital Heart Disease Adjustment for Risk Method (CHARM),24 haemodynamic vulnerability score, purpose of catheterization (interventional, biopsy, or diagnostic), number of catheterization interventions, success of the procedure (defined as achievement of the original goal of the intervention), adverse events, complications during and in the 24 h following the procedure, length of hospital stay and quality of life during hospitalization.
The quality of life was assessed using the Spanish language version of the PedsQL 4.0 (validated in the country where it was applied), which comprises 23 items that assess four dimensions: physical, emotional, social and school functioning. Each dimension consists of 4–8 questions. Responses are given on a 5-point Likert scale (0 = “never a problem” to 4 = “almost always a problem”, in reference to the past month). Items are reverse-scored and linearly transformed to yield scores ranging from 0 to 100. The scores were transformed as follows: “Never” to 100 points, “Almost never” to 75, “Sometimes” to 50, “Often” to 25, and “Almost always” to 0; with higher scores indicating a better quality of life. The scale is available in different versions for specific age groups: 2–4 years (toddlers); 5–7 years (young children); 8–12 years (children); and 13–18 years (adolescents).25 We obtained a license to use the PedsQL 4.0 scale for scientific research purposes, and the parent-proxy report was used for all age ranges.
Statistical analysisIn the descriptive analysis, we calculated frequencies and percentages for categorical variables. Normality was assessed using the Kolmogorov–Smirnov test. We calculated means and standard deviations for variables that followed a normal distribution, and medians and interquartile ranges for those that did not. We evaluated differences between age groups with the χ2 or Fisher exact test, and used the nonparametric Kruskal–Wallis test for continuous variables, as applicable based on the distribution. Subsequently, we conducted a bivariate linear regression analysis comparing each dimension (physical, emotional, social, and school functioning) with each independent variable to identify candidates for the final model. We then fitted 4 multivariate linear regression models in which the overall score for the given dimension was the dependent variable to identify associated factors. Statistical significance was defined as P < .05. All statistical analyses were performed using the software Stata 16.
Ethical considerationsThe study was approved by the Institutional Ethics Committee of the Cardiovascular Foundation of Colombia, ensuring compliance with the highest ethical standards at both national and international levels. Throughout its execution, vigilance was exerted to ensure adherence the principles and regulations governing biomedical research, thus guaranteeing the integrity and wellbeing of all participants involved in the study. In particular, informed consent was obtained from all parents or legal guardians for minors, as well as assent from mature minors, when applicable. The study has ensured the confidentiality of the analysed information and adhered to ethical practices in securing participant consent.
ResultsThe study included a total of 164 patients distributed in different age groups: 20.12% aged 2–4 years, 23.17% aged 5–7 years, 29.87% aged 8–12 years, and 26.82% aged 13–18 years. The 2–4 years group had the highest proportion of female patients (63.64%). When it came to the primary diagnoses, pulmonary atresia was most prevalent in the 2–4 years group (18.75%) and the 8–12 years group (13.64%). Ductus arteriosus was diagnosed in 15.63% of patients aged 2–4 years. As for the timing, diagnosis at birth was more frequent in all age groups except for the group aged 2–4 years, in which postoperative diagnosis predominated (53.13%).
Complications during the procedure were rare (0%–2.27%), with no significant differences among the groups (P = .485). Adverse events were also rare (0%–2.94%), with no significant differences among age groups (P = .58). Complications in the first 24 h post-procedure were infrequent (0%–6.06%; P = .511). The hospital length of stay was similar across all age groups, with a median of 1 day and a range of 0–87 days, and no significant differences (P = .874). Table 1 presents these results.
Sociodemographic characteristics of patients with congenital heart diseases undergoing hemodynamic procedures by age group.
| Variable | Categories | 2−4 years (n = 33) | 5−7 years (n = 38) | 8−12 years (n = 49) | 13−18 years (n = 44) | Total | P | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |||
| Sex | Female | 21 | 63.64 | 17 | 44.74 | 26 | 53.06 | 20 | 45.45 | 84 | 51.22 | .344 |
| Male | 12 | 36.36 | 21 | 55.26 | 23 | 46.94 | 24 | 54.55 | 80 | 48.78 | ||
| Primary diagnosis | Atrial septal defect | 1 | 3.13 | 4 | 11.76 | 11 | 25 | 7 | 17.07 | 23 | 15.23 | .124 |
| Ventricular septal defect | 3 | 9.38 | 4 | 11.76 | 3 | 6.82 | 4 | 9.76 | 14 | 9.27 | ||
| Pulmonary atresia | 6 | 18.75 | 4 | 11.76 | 6 | 13.64 | 2 | 4.88 | 18 | 11.92 | ||
| Cardiomyopathy | 2 | 6.25 | 1 | 2.94 | 4 | 9.09 | 2 | 4,88 | 9 | 5.96 | ||
| Single ventricle | 3 | 9.38 | 3 | 8.82 | 5 | 11.36 | 6 | 14.63 | 17 | 11.26 | ||
| Patent ductus arteriosus | 5 | 15.63 | 4 | 11.76 | 0 | 0 | 0 | 0 | 9 | 5.96 | ||
| Other | 12 | 37.5 | 14 | 41.18 | 15 | 34.09 | 20 | 48.78 | 61 | 40.4 | ||
| Type of diagnosis | At birth | 15 | 46.88 | 19 | 55.88 | 25 | 56.82 | 24 | 57.14 | 83 | 54.61 | .804 |
| Postoperative | 17 | 53.13 | 15 | 44.12 | 19 | 43.18 | 18 | 42.86 | 69 | 45.39 | ||
| CHARM risk | 1 | 11 | 34.38 | 17 | 50 | 19 | 43.18 | 22 | 52.38 | 69 | 45.39 | .814 |
| 2 | 12 | 37.5 | 11 | 32.35 | 16 | 36.36 | 10 | 23.81 | 49 | 32.24 | ||
| 3 | 5 | 15.63 | 2 | 5.88 | 3 | 6.82 | 5 | 11.9 | 15 | 9.87 | ||
| 4 | 4 | 12.5 | 4 | 11.76 | 6 | 13.64 | 5 | 11,9 | 19 | 12.5 | ||
| Vulnerability | Yes | 0 | 0 | 3 | 8.82 | 1 | 2.27 | 2 | 4.88 | 6 | 3.97 | .49 |
| No | 23 | 71.88 | 24 | 70.59 | 36 | 81.82 | 30 | 73.17 | 113 | 74.83 | ||
| Not calculated | 9 | 28.13 | 7 | 20.59 | 7 | 15.91 | 9 | 21.95 | 32 | 21.19 | ||
| Catheterization type | Interventional | 21 | 65.63 | 18 | 52.94 | 23 | 52.27 | 18 | 43.9 | 80 | 52.98 | .527 |
| Diagnostic | 9 | 28.13 | 15 | 44.12 | 19 | 43.18 | 22 | 53.66 | 65 | 4.,05 | ||
| Biopsy | 2 | 6.25 | 1 | 2.94 | 2 | 4.55 | 1 | 2.44 | 6 | 3.97 | ||
| Number of procedures | 1 | 20 | 95.24 | 16 | 88.89 | 20 | 86.96 | 18 | 100 | 74 | 92.5 | .249 |
| 2 | 0 | 0 | 2 | 11.11 | 3 | 13.04 | 0 | 0 | 5 | 6.25 | ||
| 3 | 1 | 4.76 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1.25 | ||
| Success of procedure | Yes | 32 | 100 | 33 | 97.06 | 43 | 97.73 | 39 | 95.12 | 147 | 97.35 | .638 |
| No | 0 | 0 | 1 | 2.94 | 1 | 2.27 | 2 | 4.88 | 4 | 2.65 | ||
| Procedure complications | Yes | 0 | 0 | 0 | 0 | 1 | 2.27 | 0 | 0 | 1 | 0.66 | .485 |
| No | 32 | 100 | 34 | 100 | 43 | 97.73 | 41 | 100 | 150 | 99.34 | ||
| Adverse events | No | 32 | 100 | 33 | 97.06 | 43 | 97.73 | 41 | 100 | 149 | 98.68 | .580 |
| Yes | 0 | 0 | 1 | 2.94 | 1 | 2.27 | 0 | 0 | 2 | 1.32 | ||
| Complications in 24 h | No | 31 | 100 | 31 | 93.94 | 42 | 97.67 | 40 | 97.56 | 144 | 97.3 | .511 |
| Yes | 0 | 0 | 2 | 6.06 | 1 | 2.33 | 1 | 2.44 | 4 | 2.7 | ||
| Length of stay (days) | Median (range) | 1 | 0−87 | 1 | 1−16 | 1 | 1−18 | 1 | 0−20 | 1 | 0−87 | .874 |
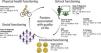
When we analysed each of the dimensions, we found that physical functioning was most impaired in these patients, with a median score of 32.14 (IQR 17.14–62.87), followed by schooling, with a median of 56 (IQR 28–88). On the other hand, emotional functioning seemed better, with a median of 62 (IQR 32–74), while scores in social functioning showed the highest quality of life, with a median of 68 (IQR 44–100). When we assessed the scores by age group, we found that the median physical functioning score was highest in children aged 8–12 years at 35.71 points (IQR 14.25–64.28), followed by the group aged 13–18 years with 34.28 points (IQR 21.42–55.71), while in the 2–4 years group it was 30 (IQR 18.57–52.85) and in the 5–7 years group29.28 (IQR 12.85–62.85) (P = .601). Similarly, the median score in the emotional functioning category was highest in the group aged 5–7 years at 69 points (IQR 48–76), followed by the 2–4 years group with 62 points (IQR 42–70), the 8–12 years group with 60 points (IQR 34–76) and, with the lowest score, the 13–18 years group with 52 points (IQR 15−70) (P = .141) (Fig. 1).
In the bivariate linear regression analysis, we found that the primary diagnosis was significantly associated with physical functioning. For instance, pulmonary atresia (P < .001) and single ventricle defect (P = .01) were associated with significant impairment in this dimension. We also found diagnoses with an impact on emotional functioning, such as single ventricle (P = .01). Likewise, the postoperative diagnosis was associated with negative coefficients (P = .01). In addition, having a greater body surface area had a negative impact on children’s emotional functioning (P = .04).
In the assessment of social functioning, we found that patient weight had a significant impact (P = .04), while other variables were not associated with quality of life in this subscale. In the analysis of school functioning, the diagnosis of single ventricle exhibited a significant association (P = .04). Similarly, patients classified as CHARM risk level 2 had poorer scores in school functioning (P = .02). Lastly, patients who experienced adverse events exhibited a marked decrease in school functioning scores (P = .03) (Table 2).
Clinical variables by dimensions of quality of life in children with congenital heart disease.
| Variable | Physical health functioning | Emotional functioning | Social functioning | School functioning | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | 95 CI | P | Coefficient | 95 CI | P | Coefficient | 95 CI | P | Coefficient | 95 CI | P | |
| Age | ||||||||||||
| 5–7 | 0.14 | −13.65 to 13.93 | 0.98 | 5.71 | −7.79 to 19.22 | 0.41 | 2.45 | −10.83 to 15.73 | 0.72 | −17.89 | −36 to 0.21 | 0.05 |
| 8–12 | 4.5 | −8.55 to 17.55 | 0.50 | −0.71 | −13.49 to 12.07 | 0.91 | 5.59 | −6.98 to 18.16 | 0.38 | −15.83 | −33.24 to 1.59 | 0.08 |
| 13–18 | 5.48 | −7.87 to 18.82 | 0.42 | −10.55 | −23.61 to 2.52 | 0.11 | −10.85 | −23.7 to 2.01 | 0.10 | −11.14 | −28.83 to 6.55 | 0.22 |
| Sex | ||||||||||||
| Male | −0.15 | −9.18 to 8.88 | 0.97 | −3.93 | −12.91 to 5.05 | 0.39 | −4.46 | −13.33 to 4.41 | 0.32 | −5.44 | −16.01 to 5.13 | 0.31 |
| Primary diagnosis | ||||||||||||
| Ventricular septal defect | −14.08 | −32.88 to 4.73 | 0.14 | −13.94 | −33.15 to 5.26 | 0.15 | 7.48 | −11.88 to 26.85 | 0.45 | −2.83 | −25.18 to 19.52 | 0.80 |
| Pulmonary atresia | −27.47 | −44.92 to −10.01 | 0.00 | −9.64 | −27.47 to 8.19 | 0.29 | −2.64 | −20.62 to 15.33 | 0.77 | −9.16 | −32.03 to 13.7 | 0.43 |
| Cardiomyopathy | −6.2 | −28.01 to 15.61 | 0.58 | −18.09 | −40.37 to 4.19 | 0.11 | 3.47 | −18.99 to 25.93 | 0.76 | −9.21 | −35.08 to 16.65 | 0.48 |
| Single ventricle | −24.82 | −42.56 to −7.07 | 0.01 | −26.32 | −44.45 to −8.2 | 0.01 | −17.50 | −35.77 to 0.77 | 0.06 | −21.76 | −42.87 to −0.65 | 0.04 |
| Patent ductus arteriosus | −11.91 | −33.72 to 9.9 | 0.28 | −7.20 | −29.48 to 15.08 | 0.52 | 03.02 | −19.43 to 25.48 | 0.79 | 5.57 | −24.54 to 35.67 | 0.72 |
| Other | −12.24 | −25.82 to 1.33 | 0.08 | −6.94 | −20.8 to 6.92 | 0.32 | −5.82 | −19.8 to 8.15 | 0.41 | −9.46 | −25.86 to 6.95 | 0.26 |
| Type of diagnosis | −13.82 | −22.81 to −4.84 | 0.00 | −11.59 | −20.85 to −2.33 | 0.01 | −9.05 | −18.35 to 0.24 | 0.06 | −10.71 | −21.76 to 0.34 | 0.06 |
| Postoperative | −3.81 | −14.35 to 6.73 | 0.48 | −6.74 | −17.46 to 3.97 | 0.22 | −5.68 | −16.37 to 5 | 0.30 | −12.86 | −25.5 to −0.23 | 0.05 |
| CHARM risk | ||||||||||||
| 2 | 9.38 | −0.99 to 19.75 | 0.08 | 4.04 | −6.79 to 14.87 | 0.46 | 5.92 | −4.75 to 16.58 | 0.28 | 0.93 | −11.8 to 13.67 | 0.89 |
| 3 | −1.1 | −16.91 to 14.72 | 0.89 | 6.90 | −9.61 to 23.42 | 0.41 | −13.87 | −30.13 to 2.4 | 0.09 | −22.69 | −42.23 to −3.14 | 0.02 |
| 4 | −12.95 | −27.34 to 1.43 | 0.08 | −5.12 | −20.14 to 9.9 | 0.50 | −2.11 | −16.9 to 12.69 | 0.78 | −12.92 | −29.7 to 3.85 | 0.13 |
| Vulnerability | ||||||||||||
| No | 03.07 | −20.83 to 26.96 | 0.80 | −9.94 | −34.06 to 14.18 | 0.42 | −7.20 | −31.3 to 16.91 | 0.56 | 17.42 | −10.02–44.87 | 0.21 |
| Not assessed | 7.62 | −17.75 to 32.99 | 0.55 | −13.48 | −39.09 to 12.13 | 0.30 | −12.98 | −38.58–12.62 | 0.32 | 19.49 | −9.73 to 48.71 | 0.19 |
| Catheterization type | ||||||||||||
| Diagnostic | −1.61 | −11.14 to 7.93 | 0.74 | −6.79 | −16.37 to 2.8 | 0.16 | −1.78 | −11.39 to 7.84 | 0.72 | 6.10 | −5.33 to 17.53 | 0.29 |
| Biopsy | 5.87 | −18.3 to 30.04 | 0.63 | −5.86 | −30.16–18.44 | 0.63 | 11.84 | −12.53 to 36.22 | 0.34 | 10.67 | −17.13 to 38.46 | 0.45 |
| Number of procedures | ||||||||||||
| 2 | −21.44 | −47.71 to 4.82 | 0.11 | −4.78 | −31.47 to 21.91 | 0.72 | 18.62 | −8.17 to 45.41 | 0.17 | −15.82 | −45.96 to 14.32 | 0.30 |
| 3 | −16.87 | −74.1 to 40.36 | 0.56 | −44.38 | −102.53 to 13.78 | 0.13 | −9.78 | −68.15–48.58 | 0.74 | 44.18 | −21.29 to 109.65 | 0.18 |
| Success of procedure | 11.51 | −17.3–40.33 | 0.43 | 4.76 | −24.42 to 33.93 | 0.75 | −5.68 | −34.87 to 23.51 | 0.70 | 28.89 | −3.99 to 61.77 | 0.09 |
| Procedure complications | −13.8 | −70.93 to 43.33 | 0.63 | −19.76 | −77.46 to 37.94 | 0.50 | −33.69 | −91.25 to 23.86 | 0.25 | 58.88 | −6.12 to 123.87 | 0.08 |
| Adverse events | 18.96 | −21.49–59.4 | 0.36 | 17.87 | −23.03 to 58.77 | 0.39 | −0.54 | −41.56 to 40.48 | 0.98 | −50.18 | −96.06 to −4.29 | 0.03 |
| Complications in 24 h | −13.27 | −42.03 to 15.48 | 0.36 | −16.46 | −45.22 to 12.3 | 0.26 | 5.32 | −23.51 to 34.15 | 0.72 | −0.11 | −33.16 to 32.94 | 1.00 |
| Length of stay (days) | −0.03 | −0.63 to 0.57 | 0.93 | −0.07 | −0.67 to 0.53 | 0.82 | 0.00 | −0.6 to 0.6 | 1.00 | 0.14 | −0.56 to 0.83 | 0.69 |
| Body surface area | 08.06 | −2.93 to 19.06 | 0.15 | −11.52 | −22.4 to −0.64 | 0.04 | −10.23 | −21.02 to 0.55 | 0.06 | 3.47 | −9.88 to 16.82 | 0.61 |
| Weight | 0.14 | −0.1 to 0.39 | 0.25 | −0.19 | −0.43 to 0.05 | 0.12 | −0.26 | −0.5 to −0.02 | 0.04 | 0.00 | −0.29 to 0.29 | 0.99 |
The multivariate analysis identified associations between various independent variables and physical functioning in children with congenital heart disease. Patients with pulmonary atresia had lower scores in this dimension (P = .048). When it came to emotional functioning, patients with a single ventricle had lower scores (P = .035) indicative of substantial impairment in this dimension. Conversely, patients with a history of cardiovascular procedures exhibited greater impairment on social functioning (P = .047) and school functioning (P = .048). Furthermore, age was associated with significantly lower scores in the groups aged 5–7 years (P = .016) and 8–12 years (P = .024). Furthermore, a CHARM risk classification of 3 was significantly associated with lower scores in school functioning (P = .01). Last of all, the presence of adverse events was also associated with lower scores in school functioning (P = .03) (Fig. 2).
DiscussionCongenital heart disease constitutes a significant global health challenge and particularly affects the paediatric age group. Advancements in cardiovascular knowledge and therapeutic strategies have improved the management and prognosis of CHD substantially, and mortality rates have declined sharply in recent decades. Nevertheless, addressing the multifaceted needs of CHD patients, particularly in regard to their HRQoL and psychosocial wellbeing, remains a complex endeavour. The findings of this study shed light on the complex relationship between various dimensions of HRQoL in paediatric patients with CHD. Notably, the variation among different age groups underscores the multifaceted nature of HRQoL assessment in this population.
Physical functioning emerged as the most affected dimension across all age groups, highlighting the significant impact of CHD on daily activities and mobility in paediatric patients.26 This finding underscores the need for personalised interventions to address the unique physical challenges faced by these individuals,27 as children with congenital heart diseases do not necessarily have to avoid physical activity. In fact, specific cardiac rehabilitation programs may be advisable in many cases to improve symptoms.28 On the other hand, the dimension with the highest levels of quality of life was social functioning. These findings likely reflect the significant medical advancements made in the detection and management of CHD in recent decades, encompassing areas such as diagnosis, neonatal cardiac surgery, paediatric intensive care and cardiac catheterization, among others.29
Similarly, emotional functioning exhibited significant variation across age groups, with higher median scores in younger children compared to adolescents. Children and adolescents with CHD have different experiences compared to their same-age peers, as they face restricted aspirations and may be dependent on their parents. They feel powerless and concerned about the debilitating physical limitations of their heart condition.30 This disparity underscores the evolving psychosocial needs of paediatric patients with CHD throughout childhood and adolescence and warrants the implementation of age-appropriate support mechanisms and psychological interventions.31
The specific diagnosis has a significant impact on the quality of life in children, and pulmonary atresia was a diagnosis that affected physical functioning, in line with the findings of Ekman-Joelsson et al., who found a higher level of psychosomatic complaints in regard to personal quality of life.32 Conversely, there is evidence of a statistically significant association with age and sex in patients with a single ventricle.33 Also in agreement with the literature, the dimensions of quality of life whose impairment was associated with single ventricle in the bivariate analysis were physical, emotional and school functioning.34 However, in the adjusted model, the association with emotional functioning was the only one that remained significant. This finding was consistent with several previous studies in paediatric patients with a single ventricle, which have reported psychological issues such as anxiety, depression and behavioural symptoms or disorders. We ought to highlight that despite these consistent findings, the results of a recent meta-analysis were inconclusive.35
Additionally, the analysis revealed significant associations between specific clinical variables and HRQoL dimensions. Notably, a history of previous cardiovascular procedures emerged as a significant predictor of HRQoL outcomes. This finding underscores the enduring impact of past medical interventions on the overall wellbeing of paediatric patients with congenital heart disease. Furthermore, the presence of adverse events was identified as a significant determinant of school functioning, suggesting that children with CHD are at risk of experiencing deficits in neurological development and cognitive impairments in educational outcomes.36 Thus, it is evident that children with congenital heart disease may require additional support or tutoring at various stages of schooling to mitigate the potential difficulties arising from their medical condition.36
This study evinced the association between various dimensions of HRQoL in paediatric CHD patients, highlighting the importance of personalised interventions to address their unique physical and emotional challenges. The findings emphasize the ongoing need for age-appropriate support resources and psychological interventions to meet the evolving psychosocial needs of these patients throughout childhood and adolescence. Lastly, this study contributes to our understanding of the complexities of CHD management and the ongoing efforts required to optimize outcomes and enhance the overall wellbeing of affected individuals.
The limitations of this study include the lack of control over external or confounding variables that were not measured (such as the complexity of congenital heart disease), which could limit the ability to establish causal relationships between the studied variables and the patients’ quality of life. We also did not use the cardiac module of the PedsQL. In addition, the questionnaire measures quality of life over the past month, which carries a risk of information bias by not recording specific data in real time. However, congenital heart disease is a chronic condition, and the assessment of quality of life continues to be relevant despite this potential limitation.
ConclusionDespite significant advancements in the management of CHD, challenges persist in assessing the HRQoL of affected children. This study revealed a varying impact on different age groups, with significant associations between specific diagnoses, such as pulmonary atresia and single ventricle, and decreased HRQoL scores in the physical and emotional dimensions. Furthermore, adverse events and certain clinical variables were significantly associated with impaired social and school functioning, emphasizing the need for comprehensive care strategies to address the multifaceted challenges faced by children with CHD who undergo cardiac catheterization.
Author contributionsMaricel Licht-Ardila participated in the conception and execution of the study, contributed to the drafting of the manuscript and its subsequent revisions, and approved the final version for publication.
Alexandra Hurtado-Ortiz participated in the conception and execution of the study, contributed to the drafting of the manuscript and its subsequent revisions, and approved the final version for publication.
Fabián Manrique-Hernández participated in the conception and execution of the study, contributed to the drafting of the manuscript and its subsequent revisions, and approved the final version for publication.
Justo José Santiago Peña participated in the conception and execution of the study, contributed to the drafting of the manuscript and its subsequent revisions, and approved the final version for publication.
Declaration of Generative AI and AI-assisted technologies in the writing processNo artificial intelligence was used.
FundingThe authors declare that no external funding or additional collaborators were involved in this study.