Infectious endocarditis (IE) is a serious disease with a poor prognosis.1 In developed countries, it occurs mainly in patients with congenital heart disease (CHD) requiring complex surgical procedures and placement of prostheses, among other factors.2,3
The main challenge in the management of IE in patients with CHD is its detection, due to its nonspecific manifestations, the limitations of the modified Duke criteria, the high frequency of negative blood culture results and the low sensitivity of echocardiography, both transthoracic (TTE) and transoesophageal (TOE).2,4
Recently, there has been an increase in the use of 18F-fluorodeoxyglucose (18F-FDG) positron-emission tomography)/computed tomography (PET/CT) for diagnosis and follow-up of certain infectious diseases, including IE in patients with CHD.5
The aim of this article is to describe our experience in a department of paediatric cardiology with the use of PET/CT in the diagnosis and follow-up of IE in children with surgically corrected CHD.
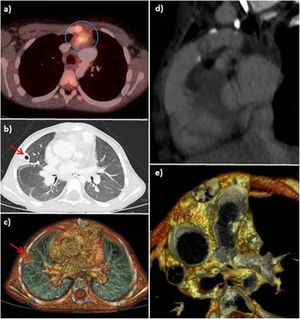
Case 1. Girl aged 4 years that had underwent the Rastelli procedure and implantation of a bovine jugular vein (BJV) graft. She developed sepsis caused by Staphylococcus aureus with evidence of a pulmonary lesion in the conventional CT scan, which was interpreted as necrotic pneumonia. The PET/CT scan detected hypermetabolic activity in the pulmonary artery graft (Fig. 1). After treatment, the patient developed symptoms again; the blood culture results were negative, but since the follow-up PET/CT scan revealed abnormalities, the final diagnosis was IE relapse, and stepwise treatment proved effective.
(a) Axial PET/CT image showing focal FDG uptake in the pulmonary graft (circle). (b) Chest CT, lung window. (c) 3D volume rendering image showing a peripheral cavitary lesion (red arrow) in the upper right lobe. (d) and (e) The retrospective interpretation of the CT scan revealed a nodular lesion suggestive of a thrombus/vegetation in the lumen of the pulmonary conduit.
Case 2. Boy aged 5 years treated with the Rastelli procedure and a BJV graft. The patient presented with persistent fever, with no features of IE in the echocardiogram. The PET/CT scan detected hypermetabolic activity in the BJV graft, which worsened despite adequate treatment and required surgical replacement of the conduit.
Case 3. Boy aged 8 years operated of tetralogy of Fallot who required percutaneous stent implantation. The patient developed febrile episodes with negative blood cultures and inconclusive findings in the echocardiogram; the PET/CT scan evinced accumulation of 18F-FDG in the stent area. The patient had an optimal outcome after antibiotherapy.
Case 4. Patient operated of truncus arteriosus type 1. He had episodes of high fever and positive blood culture results and a pedunculated lesion in the conventional CT scan in the area of the BJV graft. The PET/CT scan found tracer uptake in the area, but also a peripheral pulmonary lesion secondary to embolism that had not been detected in the conventional CT scan. After appropriate antibiotherapy, the imaging features of inflammatory activity disappeared (Table 1).
Main clinical data.
| Case | Sex | CC | Age at surgical correction | Procedure | Stents/age | Blood culture | TTE/TOE | Initial diagnosis of IE | IE after PET/CT |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | D-TGA, VSD, PS | 20m | Rastelli, pulmonary BJV graft | Ductal/1 m | S. aureus | Negative | Possible: BC major criterion + fever | Definite |
| 2 | M | LVOTO, HAA, CoA | 9m | Rastelli, pulmonary BJV graft | Aortic/3 m | S. constellatus | Thickened BJV graft | Possible: BC minor criterion + fever | Definite |
| LPAB/8 m | |||||||||
| RPAB /14 m | |||||||||
| 3 | M | Tetralogy of Fallot | 8m | VSD closure, transannular patch | LPAB /4 y 8 m | Negative | Negative | Rejected but strongly suspected: fever | Possible |
| RPAB /7 y 11 m | |||||||||
| 4 | M | CAT 1 | 4m | VSD closure, pulmonary BJV graft | None | S. epidermidis | Thickened BJV graft | Possible: BC minor criterion + fever | Definite |
BC, blood culture; BJV, bovine jugular vein; CAT 1, common arterial trunk type 1; CHD, congenital heart disease; CoA, coarctation of the aorta; D-TGA, dextro-transposition of the great arteries; F, female; HAA, hypoplastic aortic arch; IE, infectious endocarditis; LPAB, left pulmonary artery branch; LVOTO, left ventricular outflow tract obstruction; m, months; M, male; PS, pulmonary stenosis; RPAB, right pulmonary artery branch; TTE/TOE, transthoracic/transoesophageal echocardiography; VSD, ventricular septal defect; y, years.
Since PET/CT offers a high sensitivity (91%) and specificity (97%) in the diagnosis of IE in valve prostheses and intracardiac devices, it is particularly useful in paediatric patients with CHDs in who both anatomical complexity and the presence of cardiac grafts complicate and often delay the diagnosis of IE.1
It is particularly important to interpret PET/CT findings cautiously in patients that have recently undergone heart surgery, as postoperative inflammation at the surgical site can result in nonspecific uptake of 18F-FDG. Other pathological conditions that may mimic the pattern of focal increased 18F-FDG uptake are active thrombi, soft plaque atherosclerosis, vasculitis, primary cardiac tumours, cardiac metastases and foreign body reactions.2
Although the number of patients in the case series was limited, the use of PET/CT in these cases was essential for diagnosis, making evident its value by decreasing the frequency of missed diagnosis of IE: 3 of the 4 cases, initially classified as “possible” applying the Duke criteria, were reclassified to “definite”, and the fourth one, in which IE was initially “rejected”, was reclassified as “possible”.4 In addition, the PET/CT scan detected septic pulmonary emboli in 2 of the patients, something that was only possible thanks to the use of this technique. Although the prognosis of IE is poor, its early detection with the use of PET/CT identification allowed correct management that achieved favourable outcomes in every patient. We ought to highlight that in these 4 patients with right-sided IE, the infection was found in the BJV conduit, an aspect that was consistent with the previous literature.3 The mechanisms involved in the increased incidence of endocarditis in these grafts could be related to late tissue degeneration, turbulent flow, increased thrombogenicity or an intrinsic increased susceptibility of these conduits to infection.3
In this article, we share evidence that supports the importance of this imaging technique in the diagnosis of IE in patients with CHD. We believe that in the field of paediatric cardiology, PET/CT is a reliable technique to rule out or confirm IE, detect foci of septic embolism and assess the response to antibiotherapy.
Please cite this article as: Walter C, Zuccarino F, Carretero Bellón JM. Papel de la PET/TC en el diagnóstico de endocarditis infecciosa en pacientes con cardiopatía congénita. An Pediatr (Barc). 2022;96:260–263.