Foetal life takes place in a milieu that is hypoxic relative to the extrauterine environment. During embryogenesis, oxygen saturation (SatO2) ranges from 15% to 20%, and in foetogenesis it increases to 45–55%. Hypoxaemia stimulates angiogenesis through direct activation of hypoxia-inducible factor alpha (HIFα) and indirectly through vascular endothelial growth factors (VEGFs) involved in vascular and tissue development. Thus, hypoxaemia is an essential morphogen for development during foetal life. Preterm birth alters this delicate regulatory mechanism and the development and differentiation of tissues overall, but especially in the lung, intestine, retina and central nervous system.
Current recommendations for postnatal stabilisationThe onset of ventilation involves an abrupt increase in oxygen availability in the neonatal system associated with a physiological oxidative stress response that activates the enzymatic systems needed for postnatal adaptation. However, preterm newborns often require active resuscitation interventions, including oxygen supplementation. Excessive oxygen delivery may lead to hyperoxia associated with oxidative stress, while insufficient delivery may result in cardiac, haemodynamic and neurologic disturbances.1
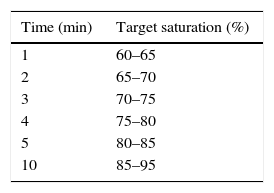
The 2015 guidelines of the International Liaison Committee on Resuscitation (ILCOR) recommend the use of room air for the resuscitation of full-term newborns, maintaining the recommendations of the ILCOR 2010 guidelines. When it comes to preterm newborns less than 35 weeks’ gestational age, they recommend the use of initial fractions of inspired oxygen (iFiO2) between 0.21 and 0.3, to be titrated in each individual based on the changes in saturation with the goal of achieving the preductal SpO2 ranges found in the assessment of newborns delivered vaginally at term and at sea level. The 2015 ILCOR guidelines do not recommend initiating stabilisation with an iFiO2 greater than 0.65 under any circumstances2 (Table 1). The impact of this recommendation is unknown, and this is acknowledged as one of the most relevant knowledge gaps in modern neonatology considering that there are 15 million preterm births a year and more than 50% of these newborns receive oxygen supplementation.
Target saturations during stabilisation expressed in minutes after birth recommended by the 2015 ILCOR guidelines.2
| Time (min) | Target saturation (%) |
|---|---|
| 1 | 60–65 |
| 2 | 65–70 |
| 3 | 70–75 |
| 4 | 75–80 |
| 5 | 80–85 |
| 10 | 85–95 |
The most recent evidence available puts into question the management of oxygen supplementation in the delivery recommended by the 2015 ILCOR guidelines. A systematic review from 2016 analysed the most relevant clinical outcomes—including death, bronchopulmonary dysplasia and intraventricular haemorrhage—in eight randomised controlled trials published to date that included infants born before 29 weeks’ gestation and compared stabilisation with iFiO2 of 0.3 or lower (n=251) and iFiO2 of 0.6 or greater (n=258).3 The study did not find differences between the two groups for the primary outcome, which was death before hospital discharge. However, when the analysis was restricted to trials that were masked for FiO2 manipulation, mortality was lower in the group with iFiO2 of 0.3 or lower (relative risk [RR], 0.46; 95% confidence interval [CI], 0.23–0.92). Conversely, when the analysis was restricted to unmasked trials, the results supported the use of iFiO2 of 0.6 or greater (RR, 1.94; 95% CI, 1.02–3.68). The unmasked trials included the TO2RPIDO 1 study, which compared resuscitation with iFiO2 of 0.21 versus 1.0. This study showed a significantly higher mortality in infants born before 29 weeks’ gestation resuscitated with an iFiO2 of 0.21 (18.9%) compared to those resuscitated with an iFiO2 of 1.0 (5.9%). The number of patients recruited in this study was notably greater than in other studies, so this study had a compelling weight in the final results of the statistical analysis.3 Recently, the Canadian Neonatal Network published a retrospective cohort study comparing mortality in preterm infants born at 27 or fewer weeks’ gestation before and after 2006, when the resuscitation protocols in the delivery room were modified and the use of iFiO2 was generally changed from 1.0 to .21. The adjusted odds ratio for the primary outcome of death or severe neurological impairment was significantly higher for the group with room air resuscitation (AOR, 1.36; 95% CI, 1.11–1.66).4
Interpreting these results poses serious difficulties, as the studies had very different designs and were conducted over an extended period of time. Thus, in studies conducted more recently, researchers were familiar with the practice of oxygen supplementation guided by pulse oximetry, used target saturations derived from studies in well-adapted patients and had experience in the customised titration of FiO2. Conversely, researchers in earlier studies lacked this information and still faced a learning curve. Another possible explanation would be a type 1 error, considering the weak statistical significance of the outcome differences between the masked and the unmasked studies.
Target saturations in the delivery roomNo data are available to assess whether adherence to the target saturations proposed by the ILCOR are associated with improvements in mortality and morbidity. This is another knowledge gap that requires exhaustive investigation in upcoming years. A study presented recently at the 2016 European Association for Paediatric Research (EAPS) Congress summarised the clinical outcomes (morbidity and mortality) of reaching or failing to reach target saturations in preterm infants by the minute-to-minute analysis of SatO2 and FiO2 in 706 patients included in eight randomised trials that compared resuscitation with high versus low FiO2.5 The primary clinical outcome was the correlation between SatO2 at 5min and death and/or intraventricular haemorrhage (IVH) with a grade 3 or greater. Interestingly, only 23% of the patients achieved the expected target saturations (85% at 5min), while 55% reached lower saturations and 22% amply overshot the target. The most relevant finding was that the patients that failed to reach the 85% target were more likely to die (OR, 1.5; 95% CI, 1.1–2.6) and/or to develop IVH (OR, 2.4; 95% CI, 1.2–4.2). The risk of both death and IVH increased with each additional minute that it took patients to reach the target saturation. We ought to highlight that increases in heart rate in these patients did not guarantee a favourable clinical outcome.
The analysis did not establish whether the higher frequency of poor outcomes in the patients that did not reach target saturations was due to insufficient oxygen supplementation or to underlying critical conditions that prevented these patients from achieving stability with the established interventions.
ConclusionsOxygen supplementation in extremely preterm newborns remains an uncharted territory. We have yet to determine the optimal initial FiO2, the target saturations throughout stabilisation, and the air/oxygen mixer mode settings required to achieve adequate stabilisation. Until we obtain results from the large international studies that are currently underway, it may be convenient to initiate reanimation with higher FiO2 settings (0.4), or, if the patient does not respond adequately, to adjust flow more rapidly (intervals of 20–30% every 10–15s) with the purpose of optimising cardiac output and achieving haemodynamic stability. Perhaps the failure to achieve established SatO2 targets could serve as a clinical indicator for the identification of high-risk patients that require even more careful monitoring.
FundingThe author has obtained support through the RETICS, with funding from the National Plan for R&D&I 2008–2011, the General Subdirectorate for the Evaluation and Promotion of Research of the Instituto de Salud Carlos III, and the European Regional Development Fund RD12/0026.
Please cite this article as: Vento M. Aporte de oxígeno en la reanimación neonatal. An Pediatr (Barc). 2017;86:1–3.