Retinopathy of prematurity (ROP) is characterised by insufficient vascular development in the retina, and requires early treatment to avoid visual disability in severe cases. ROP is currently the second leading cause of preventable child blindness in the world.
Patients and methodsThis was an observational, retrospective, case-control study including 233 preterm infants examined between 1999 and 2019.
ResultsPostnatal weight gain in the first 4 weeks of life, birth weight, gestational age, mechanical ventilation, transfusion, presence of sepsis, persistence of arterial ductus, necrotising enterocolitis, intraventricular haemorrhage, or periventricular leukomalacia were found to be significantly different between the ROP groups requiring and not requiring treatment. The mean postnatal weight gain in the ROP group not requiring treatment was 12.75 ± 5.99 g/day, whereas it was 9.50 ± 5.45 g/day in the ROP group requiring treatment. The risk of developing ROP that required treatment decreased with an increase in weight gain. The risk reduction was 2.76%–8.35% in preterm infants gaining 10 g/day, and 7.17%–12.76% in infants gaining 20 g/day.
ConclusionsThe risk of developing ROP requiring treatment decreased with increasing weight gain in the first 4 weeks of life. This was applicable in infants with postnatal weight gain ≥14 g/day. However, gestational age, birth weight, time of mechanical ventilation, and comorbidity should be taken into account when evaluating the risk of ROP requiring treatment.
La retinopatía del prematuro (ROP) se caracteriza por el desarrollo vascular insuficiente en la retina que, en los casos severos precisa tratamiento precoz para evitar secuelas visuales. Es actualmente la segunda causa mundial de ceguera infantil prevenible.
Pacientes y métodosEstudio observacional, retrospectivo, de casos-controles sobre 233 recién nacidos prematuros explorados entre 1999−2019.
ResultadosLa ganancia de peso postnatal en las primeras 4 semanas, el peso al nacer, la edad gestacional, la ventilación mecánica, las transfusiones recibidas y la presencia de sepsis, ductus arteriovenoso persistente, enterocolitis necrotizante, hemorragia intraventricular o leucomalacia periventricular, mostraron diferencias significativas entre el grupo de ROP no susceptible de tratamiento frente al grupo candidato a tratamiento. La ganancia ponderal media fue 12,75 ± 5,99 g/día en el grupo no susceptible de tratamiento y 9,50 ± 5,45 g/día en el susceptible de tratamiento. El riesgo de ROP candidata a tratamiento se redujo progresivamente con el aumento de ganancia ponderal. La reducción del riesgo fue de 2,76–8,35% en ganancias de 10 g/día, y alcanza el 7,17–12,76% en ganancias de 20 g/día.
ConclusionesEl riesgo de presentar ROP severa candidata a tratamiento disminuye con el aumento de la ganancia de peso postnatal en las primeras 4 semanas. Esta relación se mantiene en ganancias de peso >14 g/día. Sin embargo, se deben tener en cuenta la edad gestacional y peso al nacer del recién nacido, la duración de la ventilación mecánica y su comorbilidad para la evaluación global del riesgo de ROP que precisa tratamiento.
Retinopathy of prematurity (ROP) is a disease with a high prevalence in infants and is currently the second leading cause of blindness in infancy worldwide.1 It is characterised by delayed retinal vascular growth and, in the plus disease stage, rapidly progressing venous dilatation and arteriolar tortuosity of the posterior retinal vessels. The international classification of ROP comprises 5 stages of increasing severity ranging from mild anomalies in the peripheral vascular vessels in stage 1, with development of a ridge in the demarcation line in stage 2 and extraretinal neovascularization in stage 3, to partial or total retinal detachment in stages 4 and 5.2
The vascularization of the retina starts in week 16 of gestation and is completed between weeks 36 and 40. The development of ROP depends of intrinsic disease factors as well as iatrogenic factors.3 Some of these factors are birth at low gestational age, low birth weight, oxygen therapy, bronchopulmonary dysplasia, sepsis, intraventricular haemorrhage, necrotising enterocolitis and anaemia.4,5 The immaturity of newborn infants (based on low birth weight and low gestational age) and prolonged mechanical ventilation are the main risk factors for the development of ROP and the advanced stages of the disease.6,7 Other factors related to the postnatal development of the preterm infant, such as weight gain in the first 4 weeks post birth, have been growing in importance in the algorithms used for early detection of ROP.6,8,9
The levels of vascular endothelial growth factor (VEGF), involved in vascular development, are strongly associated with the levels of insulin-like growth factor 1 (IGF-1). Thus, prolonged deficits of IGF-1 in preterm infants and poor postnatal weight gain are associated with an increased risk of severe ROP.4,5,10,11
Filho et al.5 established a threshold for postnatal weight gain of 7.28 g/day, below which the risk of ROP increased significantly. However, in recent years, due to improvements in nutritional interventions and mechanical ventilation in the intensive care setting, the incidence of extremely low neonatal weight gain has decreased, with the exception of infants with multiple comorbidities.12–14 As a consequence, it is important to determine the impact of intermediate and high neonatal weight gain on ROP.
The aim of this study was to analyse the impact of neonatal weight gain in the first 4 weeks post birth on ROP requiring treatment and its protective effect against the development of ROP.
Patients and methodsThe study was approved by the Biomedical Research Ethics Committee of Andalusia and conducted in adherence with the ethical principles of the Declaration of Helsinki. All the clinical data were anonymised and collected after obtaining the informed consent of the parents or legal guardians of each participant in the study.
SampleWe conducted a retrospective, observational case-control study in 233 preterm infants assessed between 1999 and 2019 at the Hospital San Cecilio in Granada, Spain. The sample included a single eye per participant in the study.
The inclusion criteria were1: infants born with a birth weight of less than 1500 g at 32 or fewer weeks of gestation,2 with a birth weight between 1501 and 2000 g born at 32 or more weeks of gestation that required supplemental oxygen for more than 72 h of considered unstable by the neonatologist in charge at the intensive care unit (apnoea, neonatal acidosis, death of a twin, intraventricular haemorrhage, patent ductus arteriosus, sepsis, necrotising enterocolitis or neonatal surgery)3 and preterm infants that underwent evaluation at 4 weeks post birth and at least 3 evaluations in total. The exclusion criteria were1: inability to perform the evaluation at week 4 post birth or completion of follow-up before 4 weeks post birth (follow-up performed until completion of retinal vascularization, 45 weeks of postmenstrual age in absence of pre-plus disease, 36 weeks with vascularization in zone III without features of ROP, or regression of ROP with certainty that the disease will not recur),2 lens opacity3 and nonphysiological weight gain (e.g. hydrocephalus).
Clinical evaluationAll patients were examined by the same paediatric ophthalmologist on the fourth week after birth with pharmacological mydriasis and topical anaesthesia using a 20-diopter condensing lens (which provides a field of view of approximately 8 disc diameters, equivalent to 45°). The variables collected in each examination were: ROP stage based on the International Classification of Retinopathy of Prematurity,2 the zone of retinal involvement (I–III), the extent of involvement by clock hour and the presence of pre-plus and plus disease. The patients underwent examinations every 2 weeks until the retina was fully vascularised, except in patients with pre-plus or plus disease or with incomplete vascularization in zone I or posterior zone II or with stage 3 disease in any zone. These patients were examined weekly.
The dependent variable was ROP requiring treatment. The independent variables were the birth weight (in g), weeks of gestation, ROP stage,2 weight gain in the first 4 weeks post birth expressed as the mean g/day, duration of mechanical ventilation (days), maximum fraction of inspired oxygen (FiO2), presence/absence of sepsis, patent ductus arteriosus, bronchopulmonary dysplasia, intraventricular haemorrhage, periventricular leukomalacia or necrotising enterocolitis, number of transfusions received and Apgar score.15
Statistical analysisThe statistical analysis was performed with the software Statistical Package for Social Sciences (SPSS) version 25.0 (IBM Corp., Armonk, New York, United States). We performed a descriptive analysis of the study variables and evaluated the assumption of the normality of the distribution with the Kolmogorov–Smirnov test.
We compared variables by means of the Mann–Whitney U test and the χ2 test. We developed a predictive model by means of binary logistic regression, assessing the association between the dependent variable (ROP requiring treatment) and the weight gain in the first 4 weeks post birth as well as all the other independent variables previously mentioned.
ResultsDescriptive analysisThe study included 233 newborn infants. A total of 688 preterm infants were evaluated, of who we excluded 455 based on the established criteria.
We found statistically significant differences in the weight gain in the first 4 weeks post birth, birth weight, weeks of gestation, duration of mechanical ventilation, number of received transfusions, maximum FiO2 and presence of sepsis, patent ductus arteriosus, intraventricular haemorrhage, periventricular leukomalacia or necrotising enterocolitis, (P < .05) between the group of infants with ROP that did not require treatment and the group with ROP that required treatment. We did not find statistically significant differences (P > .05) in the presence/absence of bronchopulmonary dysplasia or the Apgar score between both groups (Tables 1–3; Fig. 1).
Descriptive study of quantitative variables under study. Mean and standard deviation, range and difference between the means (Mann–Whitney U test). Weight gain expressed in g/day and adjusted for gestational age in g/weeks/day.
| Total | ROP requiring treatment | P | ||
|---|---|---|---|---|
| No | Yes | |||
| n | 233 | 193 | 40 | |
| % | 100 | 82.8 | 17.2 | |
| Weight gain (g/day) | 12.19 ± 6.015 | 12.75 ± 5.99 | 9.50 ± 5.45 | ** |
| (range, 0.10–39.71) | (range, 0.14–39.71) | (range, 0.10–24.00) | ||
| Weight gain (g/weeks/day) | 0.422 ± 0.204 | 0.437 ± 0.201 | 0.350 ± 0.209 | * |
| (range, 0.004–1.348) | (range, 0.004–1.348) | (range, 0.004–0.899) | ||
| Weight gain (g) | 1096.33 ± 266.90 | 1129.07 ± 256.32 | 938.35 ± 263.45 | *** |
| (range, 537–1.970) | (range, 596–1970) | (range, 537–1546) | ||
| Gestational age (weeks) | 28.82 ± 1.98 | 29.11 ± 1.87 | 27.43 ± 1.91 | *** |
| (range, 24–34) | (range, 24–34) | (range, 24.70–31.15) | ||
| Duration of mechanical ventilation (days) | 9.40 ± 13.61 | 6.73 ± 8.62 | 22.41 ± 23.07 | *** |
| (range, 0–100) | (range, 0–43) | (range, 0–100) | ||
| Number of transfusions | 0.64 ± 0.67 | 0.58 ± 0.60 | 0.95 ± 0.90 | * |
| (range, 0–5) | (range, 0–4) | (range, 0–5) | ||
| Maximum FiO2 (%) | 32.58 ± 16.64 | 42.00 ± 20.96 | 31.09 ± 16.22 | * |
| (range, 21–100) | (range, 21–100) | (range, 21–100) | ||
FiO2, fraction of inspired oxygen.
Descriptive analysis of dichotomous qualitative variables under study. Percentages (%) and differences in percentages (Pearson χ2 test).
| Total | ROP requiring treatment | P | ||
|---|---|---|---|---|
| No | Sí | |||
| n | 233 | 193 | 40 | |
| % | 100 | 82.8 | 17.2 | |
| Sepsis | 39.9% | 35.23% | 62.5% | ** |
| Patent ductus arteriosus | 20.6% | 15.79% | 44.74% | *** |
| Bronchopulmonary dysplasia | 79.8% | 78.12% | 87.5% | * |
| Intraventricular haemorrhage | 14.0% | 10.5% | 31.6% | ** |
| Periventricular leukomalacia | 24.1% | 19.5% | 47.4% | *** |
| Necrotising enterocolitis | 23.6% | 18.7% | 47.5% | *** |
| ROP stage [n (%)] | No ROP | ROP 1 | ROP 2 | ROP 3 | ROP 4 | ROP 5 | Min | Max | P |
|---|---|---|---|---|---|---|---|---|---|
| 129 (55.4%) | 36 (15.5%) | 18 (7.7%) | 49 (21.0%) | 1 (0.4%) | 0 (0%) | 0 | 4 | ** | |
| 1-min Apgar [n (%)] | 0 | 1–2 | 3–4 | 5–6 | 7–8 | 9–10 | Min | Max | |
| 5 (2.1%) | 25 (10.7%) | 46 (19.7%) | 56 (24.0%) | 66 (28.3%) | 35 (15.0%) | 0 | 10 | * | |
| 5-min Apgar [n (%)] | 0 | 1–2 | 3–4 | 5–6 | 7–8 | 9–10 | Min | Max | |
| 0 | 3 | 11 | 30 | 63 | 126 | 1 | 10 | * | |
| (0%) | (1.3%) | (4.7%) | (12.9%) | (27.0%) | (54.1%) |
We found statistically significant differences in the crude and adjusted weight gain in the first 4 weeks post birth (P < .05) between the 2 groups in the subcohort obtained by excluding infants with birth weights of less than 1000 g (Table 4).
Analysis of the difference between the means in a subcohort that excluded extremely low birth weight infants (<1000 g). Mean and standard deviation, range, difference between means (Mann–Whitney U test).
| Variable | Total | Untreated ROP | Treated ROP | P |
|---|---|---|---|---|
| Weight gain (g/day) | 12.53 ± 6.31 | 12.947 ± 6.388 | 8.275 ± 3.230 | .003 |
| (range, 0.14–39.71) | (range, 0.14–39.71) | (range, 4.38–13.46) | ||
| Weight gain adjusted for gestational age (g/weeks/day) | 0.424 ± 0.213 | 0.438 ± 0.216 | 0.284 ± 0.115 | .004 |
| (range, 0.004–1.348) | (range, 0.004–1.348) | (range, 0.145–0.491) | ||
| Weight gain adjusted for birth weight (g/kg/day) | 0.010 ± 0.006 | 0.011 ± 0.006 | 0.007 ± 0.003 | .007 |
| (range, 0.0001–0.037) | (range, 0.001–0.037) | (range, 0.003–0.013) | ||
| Weight gain adjusted for gestational age and birth weight (g/kg/weeks/day) | 0.306 ± 0.166 | 0.3168 ± 0.168 | 0.200 ± 0.087 | .005 |
| (range, 0.003–1.095) | (range, 0.003–1.095) | (range, 0.085–0.356) |
Following the bivariate analysis of weight gain in the first 4 weeks post birth adjusted for the different variables under study, the statistically significant differences in weight gain between the treated ROP and untreated ROP groups remained, with no appreciable confounding effect from the remaining variables (P < .05). The crude weight gain (crude OR, 0.830; 95% confidence interval [CI], 0.831–0.960; P = .002) did not lose its statistical significance when it was adjusted for the birth weight (adjusted OR, 0.879 95% CI, 0.811–0.952; P = .002).
The full logistic regression model (which included all the independent variables with a P-value of less than 0.02 in the bivariate analysis), showed that the risk of developing ROP requiring treatment in preterm infants was indirectly proportional to the neonatal weight gain in the first 4 weeks post birth. We found that 62.8% of the variance in the development of ROP requiring or not requiring treatment was explained by the independent variables: gestational age, birth weight, duration of mechanical ventilation, number of received transfusions, maximum FiO2, presence/absence of patent ductus arteriosus, sepsis, intraventricular haemorrhage, periventricular leukomalacia or enterocolitis and Apgar score (Nagelkerke R2, 62.8%; P < .001). The OR for the weight gain in the multivariate logistic regression model was 0.797 (95% CI, 0.684–0.929; P = .004).
When it came to the preventable risk factors in the multivariate model, we found that the weight gain in the first 4 weeks post birth (P = .004) and the duration of mechanical ventilation (P = .043) alone explained 26.9% of the variance in the severity of ROP determining the need for treatment (Nagelkerke R2, 26.9%; P < .001).
Thus, the equation for the probability of developing ROP requiring treatment based on weight gain was:
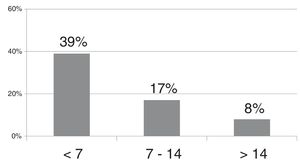
The probability of developing ROP requiring treatment based on the neonatal weight gain (g/day) in the first 4 weeks post birth, assuming that all other variables remained constant, ranged from 16.85% and 11.26% in infants gaining less than 7 g/day, from 10.51% to 6.85% in infants gaining 7–14 g/day and decreased gradually for weight gains starting from 14 g/day.
The decrease in the risk of ROP requiring treatment was of up to 8.35% for postnatal weight gains of 10 g/day and of up to 12.76% for postnatal weight gains of 20 g/day (Table 5).
Decrease in the probability of severe ROP requiring treatment based on neonatal weight gain (g/day) in the first 4 weeks post birth. Estimated risk reduction assuming all other independent factors remain constant, P < .005.
| g/day | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| Decrease in probability | 0.75%–6.34% | 1.46%–7.05% | 2.13%–7.72% | 2.76%–8.35% | 3.35%–8.94% | 3.90%–9.49% | 4.41%–10% |
| g/day | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| Decrease in probability | 4.89%–10.48% | 5.34%–10.93% | 5.76%–11.35% | 6.15%–11.74% | 6.52%–12.11% | 6.86%–12.45% | 7.17%–12.76% |
Among the independent variables under study, we found the most significant associations for the classical risk factors for ROP described in numerous previous studies: birth weight, gestational age, duration of mechanical ventilation and neonatal comorbidities.6,16,17
Birth weight and gestational age at birth are the 2 key characteristics that reflect the degree of immaturity of preterm newborn infants18 and therefore the severity of retinal vascular growth deficits. Gestational age is the most indicative factor of the time missing in vascular development to complete vascularization of the retina. The greater the retinal area, the greater the rate of vascular growth required to achieve full vascularization.19,20 The suppression of growth factors during hyperoxia and the loss of placental growth factors after birth result in delays in retinal vascular development, which will result in hypoxia when metabolic activity increases in the poorly vascularized retina and stimulate the production of proliferative growth factors like VEGF. This gives rise to an uncontrolled vascular proliferation that is responsible for the most severe stages of ROP.20
As has been done in many published screening algorithms,21,22 the most prominent of which may be the WINROP algorithm,4 we described postnatal weight gain in g/day as opposed to g/kg/day. In the previous literature, low birth weight has been associated with prenatal factors and comorbidities,23–25 as reflected in the concept of small for gestational age (SGA), which considers the weight of the patient relative to gestational age. The investigation of the pathogenesis of SGA and intrauterine growth restriction needs to take into account delays in foetal growth due to maternal, placental and foetal causes and the prioritization (mediated by IGF-1) of the development of vital organs, such as those in the nervous system.11,24 The presence of insulin-like growth factor 1 and its permissive effect on VEGF are among the most relevant factors involved in the pathophysiology of ROP.26,27 Thus, the levels of IGF-1 are substantially lower in neonates born before 28 weeks compared to full-term neonates (mainly due to the early loss of growth factors derived from the placenta).28 Hellström et al. concluded that IGF-I is at least as strong a determinant of risk for ROP as postmenstrual age at birth.29 The levels of IGF-1, which were previously used in screening algorithms like the WINROP,30 were later replaced by weight gain (g/day) due to the direct and strong association between these two factors.31,32
Given the importance of intrauterine nutrition in weight gain during the neonatal period and the decreased weight gain, poorer intrauterine growth, poorer nutritional status at 36 weeks of postmenstrual age and developmental delay observed in infants born at 30 or fewer weeks of gestation,23–25 weight gain (g/day) must always be considered in combination with gestational age, birth weight and any comorbidities present in the preterm newborn infant. We did an additional analysis of its impact taking into account gestational age and excluding extremely low birth weight (<1000 g) infants from the analysis.33
To the intrinsic factors found in preterm infants we must add the iatrogenic factors resulting from the treatments required by the immaturity of the infant or the presence of comorbidities. The most important iatrogenic risk factor in recent years in has been the duration of mechanical ventilation. Between 2009 and 2012, the BOOST II,34 SUPPORT35 and ELGAN36 clinical trials, among other studies, evinced the increased risk of severe ROP associated with high oxygen saturation values in the first weeks of life.7
Improvements in the treatment of neonatal comorbidities and intervention with use of oxygen therapy and careful monitoring of nutritional intake, especially in neonates receiving early breastfeeding, have led to an increasing trend in weight gain in everyday clinical practice.13,14 While the negative impact is most marked in cases with extremely low weight gains of less than 7 g/day, intermediate weight gains ranging from 7 and 14 g/day and especially weight gains of more than 14 g/day are still strongly, proportionally and inversely associated with the severity of ROP. Seventy-five percent of the cases with weight gains of less than 7 g/day in our study corresponded to preterm infants born before 31 weeks’ gestation, product of a multiple pregnancy or with comorbidities. There was 1 newborn infant with an extremely large weight gain (40.1 g/day) associated with hydrocephalus, which we therefore excluded from the study. The minimum weight gain was 0.10 g/day and observed in 3 infants born at 24 and 27 weeks of gestation, one product of a singleton pregnancy and 2 of a twin pregnancy, all with serious comorbidities, and with birth weights of 753, 900 and 820 g, respectively.
The leading comorbidity among those detected in the sample was bronchopulmonary dysplasia, previously described by Bancalari in 1979,37 found in 79.8% of the preterm infants in our study.
In addition to the intrinsic and extrinsic factors discussed above, Flynn et al proposed the possibility of a genetic component in ROP38 supported by evidence in different ethnic groups39 and in infants product of multiple gestations.11
There are weaknesses and limitations to our study, including those intrinsic in its retrospective design and the exposure to different oxygen therapy protocols in the years preceding and following the 2009–2012 period. We ought to highlight that in 2009, the protocol for the management of preterm infants underwent significant changes, such as the introduction of protocols for oxygen administration and the Oxygen With Love programme (OWL)7 in the hospital setting, and more thorough monitoring of weight gain in the neonatal period.31 We present the FiO2 values for the entire sample, but we were unable to analyse data on the use of non-invasive ventilation methods or the haemoglobin concentration in the periods before and after 2009. On the other hand, the monitoring and recording of nutritional intake and the type of nutrition used in each infant was not documented consistently enough to allow its analysis in this study. Images were obtained with a video camera attached to the binocular indirect ophthalmoscope,40 which allowed saving the examinations and future re-evaluation, but the resolution was not good enough to extract quality static images allowing objective measurement of vascular progression in each evaluation, like the RetCam® system does.
ConclusionsWeight gain in the first 4 weeks post birth in preterm infants is inversely correlated to the probability of developing severe ROP requiring treatment. This close association between weight gain and ROP requiring treatment is not only important in infants with low weight gains, but the risk of ROP requiring treatment also decreases gradually as weight gain increases in the first 4 weeks post birth. The probability of severe ROP can guide clinicians in clinical practice and the adaptation of follow-up schedules based on the level of risk, but it should not be considered in isolation. In addition to this probability, clinicians must consider the risk associated with the immaturity of the neonate (gestational age and birth weight), iatrogenic factors (chiefly the duration of mechanical ventilation) and any comorbidities present in the infant.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: María J Samaniego, María C. Chaves-Samaniego. Enuresis. Nuevas evidencias sobre el efecto protector de la ganancia de peso en la retinopatía del prematuro. An Pediatr (Barc). 2021;95:78–85.