Prenatal alcohol exposure is the leading preventable cause of cognitive deficit in developed countries and can lead to fetal alcohol spectrum disorder (FASD). This term encompasses a wide range of physical, mental, behavioral, and cognitive effects that result from damage caused by exposure to alcohol during intrauterine life. Alcohol consumption among the general population is common in Eastern European countries and especially among women at risk of social exclusion, who are the ones who lose or give up custody of their children. A high number of these children are adopted in Spain and many of them present neurocognitive and behavioral disorders, causing FASD to be a public health problem in our country. In many occasions this clinical spectrum is delayed or under-diagnosed due to the overlapping of neuropsychological symptoms caused by the abandonment. A neurocognitive and behavioral profile specific for FASD has not been defined and all the symptoms are common to other etiologies. The aim of this work is to review the neuropsychological profile in the diagnosis of FASD.
La exposición prenatal al alcohol es la principal causa prevenible de déficit cognitivo en los países desarrollados y puede dar lugar al trastorno del espectro alcohólico fetal (TEAF). Este término engloba una gran variedad de efectos físicos, mentales, conductuales y cognitivos que derivan del daño causado por la exposición al alcohol durante la vida intrauterina. El consumo de alcohol entre la población general es frecuente en los países de la Europa del Este y especialmente entre las mujeres en riesgo de exclusión social, que son las mayores afectadas en procesos de pérdida o renuncia de custodia de sus hijos. Un elevado número de estos niños son adoptados en España y muchos de ellos presentan alteraciones neurocognitivas y conductuales, convirtiendo el TEAF en un problema de salud pública en nuestro país. En muchas ocasiones este cuadro clínico está infradiagnosticado debido a la superposición de los síntomas neuropsicológicos causados por el abandono y la falta de apego. Hasta el momento, no se ha descrito un perfil neurocognitivo y conductual específico del TEAF y muchos de los síntomas son comunes a otras etiologías. El objetivo de este trabajo es revisar el perfil neuropsicológico en el diagnóstico de TEAF.
Neurocognitive disorders have been increasing in our society.1 One of the known causes of this increase is exposure to toxic industrial chemicals (lead, methylmercury, polychlorinated biphenyls, arsenic, toluene, fluoride), which can damage the developing brain. The current interest in establishing the impact on exposure to these substances stands in contrast to their possibly unavoidable presence in the environment. On the other hand, alcohol is one of the best-known teratogens and the main identifiable and preventable cause congenital anomalies and cognitive impairment.2
Foetal alcohol spectrum disorder (FASD) is an umbrella term that encompasses a broad spectrum of physical, mental, behavioural and cognitive abnormalities that may manifest in individuals with a history of prenatal exposure to alcohol.3,4 It is a clinical spectrum that includes foetal alcohol syndrome (FAS), partial FAS, alcohol-related neurodevelopmental disorder (ARND) and alcohol-related birth defects (ARBDs) (Table 1).5–8 Although in 2013 the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) introduced the diagnosis of neurobehavioral disorder associated with prenatal alcohol exposure (ND-PAE) to refer to neurocognitive impairment associated with maternal alcohol use during pregnancy, the specific neuropsychological profile of FASD has yet to be described.
Diagnostic categories of FASD (Hoyme, 2016).8
| I. FAS (foetal alcohol syndrome) |
| A diagnosis of FAS requires all features, A–D (with or without confirmation of prenatal alcohol exposure): |
| A. characteristic pattern of minor facial anomalies, including ≥2 of the following: |
| 1. Short palpebral fissures (≤10th percentile) |
| 2. Thin vermilion border of the upper lip (rank 4 or 5 on a racially normed lip/philtrum guide) |
| 3. Smooth philtrum (rank 4 or 5 on a racially normed lip/philtrum guide) |
| B. Prenatal and/or postnatal growth deficiency |
| 1. Height and/or weight ≤10th percentile |
| C. Deficient brain growth, abnormal morphogenesis, or abnormal neurophysiology, including ≥1 of the following: |
| 1. Head circumference ≤10th percentile |
| 2. Structural brain anomalies |
| 3. Recurrent nonfebrile seizures |
| D. Neurobehavioural impairment |
| 1. For children ≥3 years of age (a or b) |
| a. With cognitive impairment: |
| Evidence of global impairment (general conceptual ability ≥1.5 SD below the mean, or performance IQ or verbal IQ or spatial IQ ≥ 1.5 SD below the mean) |
| OR |
| Cognitive deficit in at least 1 neurobehavioural domain ≥1.5 SD below the mean (executive functioning, specific learning impairment, memory impairment or visual-spatial impairment) |
| b. With behavioural impairment without cognitive impairment: |
| Evidence of behavioural deficit in at least 1 domain ≥1.5 SD below the mean in impairments of self-regulation (mood or behavioural regulation impairment, attention deficit, or impulse control) |
| 2. For children <3 years of age: |
| Evidence of developmental delay ≥1.5 SD below the mean |
| II. PFAS (partial foetal alcohol syndrome) |
| (1) For children with documented prenatal alcohol exposure, a diagnosis of PFAS requires features A and B: |
| A. characteristic pattern of minor facial anomalies, including ≥2 of the following: |
| 1. Short palpebral fissures (≤10th percentile) |
| 2. Thin vermilion border of the upper lip (rank 4 or 5 on a racially normed lip/philtrum guide) |
| 3. Smooth philtrum (rank 4 or 5 on a racially normed lip/philtrum guide) |
| B. Neurobehavioural impairment |
| 1. For children ≥3 years of age (a or b): |
| a. With cognitive impairment: |
| Evidence of global impairment (general conceptual ability ≥1.5 SD below the mean, or performance IQ or verbal IQ or spatial IQ ≥ 1.5 SD below the mean) |
| OR |
| Cognitive deficit in at least 1 neurobehavioral domain ≥1.5SD below the mean (executive functioning, specific learning impairment, memory impairment or visual-spatial impairment) |
| b. With behavioural impairment without cognitive impairment: |
| Evidence of behavioural deficit in at least 1 domain ≥1.5 SD below the mean in impairments of self-regulation (mood or behavioural regulation impairment, attention deficit, or impulse control) |
| 2. For children <3 years of age: |
| Evidence of developmental delay ≥1.5 SD below the mean |
| (2) For children without documented prenatal alcohol exposure, a diagnosis of PFAS requires all features, A–C: |
| A. characteristic pattern of minor facial anomalies, including ≥2 of the following: |
| 1. Short palpebral fissures (≤ 10th percentile)) |
| 2. Thin vermilion border of the upper lip (rank 4 or 5 on a racially normed lip/philtrum guide) |
| 3. Smooth philtrum (rank 4 or 5 on a racially normed lip/philtrum guide) |
| B. Growth deficiency or deficient brain growth, abnormal morphogenesis, or abnormal neurophysiology: |
| 1. Height and/or weight ≤10th percentile, or, |
| 2. Deficient brain growth, abnormal morphogenesis or neurophysiology, including ≥1 of the following: |
| a. Head circumference ≤10th percentile |
| b. Structural brain anomalies |
| c. Recurrent nonfebrile seizures |
| C. Neurobehavioural impairment |
| 1. For children ≥3 years of age (a or b): |
| a. With cognitive impairment: |
| Evidence of global impairment (general conceptual ability ≥1.5 SD below the mean, or performance IQ or verbal IQ or spatial IQ ≥ 1.5 SD below the mean) |
| OR |
| Cognitive deficit in at least 1 neurobehavioral domain ≥1.5 SD below the mean (executive functioning, specific learning impairment, memory impairment or visual-spatial impairment) |
| b. With behavioural impairment without cognitive impairment: |
| Evidence of behavioural deficit in at least 1 domain ≥1.5 SD below the mean in impairments of self-regulation (mood or behavioural regulation impairment, attention deficit, or impulse control) |
| 2. For children <3 years of age: |
| Evidence of developmental delay ≥1.5 SD below the mean |
| III. ARND (alcohol-related neurodevelopmental disorder) |
| Requires features A and B (this diagnosis cannot be made definitively in children <3 years of age): |
| A. Documented prenatal alcohol exposure |
| B. Neurobehavioural impairment |
| a. With cognitive impairment: |
| Evidence of global impairment (general conceptual ability ≥1.5 SD below the mean, or performance IQ or verbal IQ or spatial IQ ≥ 1.5 SD below the mean) |
| OR |
| Cognitive deficit in at least 2 neurobehavioral domains ≥1.5 SD below the mean (executive functioning, specific learning impairment, memory impairment or visual-spatial impairment) |
| b. With behavioural impairment without cognitive impairment: |
| Evidence of behavioural deficit in at least 2 domains ≥1.5 SD below the mean in impairments of self-regulation (mood or behavioural regulation impairment, attention deficit, or impulse control) |
| IV. ARBD (alcohol-related birth defects) |
| Requires features A and B: |
| A. Documented prenatal alcohol exposure |
| B. One or more specific major malformations (demonstrated in animal models and human studies to be the result of prenatal alcohol exposure): cardiac: atrial septal defects, aberrant great vessels, ventricular septal defects, conotruncal heart defects; skeletal: radioulnar synostosis, vertebral segmentation defects, large joint contractures, scoliosis; renal: aplastic/hypoplastic/dysplastic kidneys, “horseshoe” kidneys/ureteral duplications; eyes: strabismus, ptosis, retinal vascular anomalies, optic nerve hypoplasia; ears: conductive hearing loss, neurosensory hearing loss |
IQ, intellectual quotient; SD, standard deviation.
The estimated prevalence of FASD in our region is of 9–10 cases per 1000 live births.9,10 In Europe, the estimated prevalence of FAS is of 1.4–2 per 1000 live births and the prevalence of FASD 3–5 times higher.11 A retrospective study found a prevalence of FAS in Italy of 3.7–7.4 per 1000 live births and of FASD of 20.3–40.5 per 1000 live births.12 In Spain, there are no data for the general population, but a study in children adopted from Eastern Europe, the group at highest risk, found a prevalence of FASD of 50%, similar to the prevalence reported in Sweden.13–15 There is an alarmingly high frequency of FAS in infants institutionalised in orphanages in Eastern Europe (5.1%–33.5%), where women that consume high amounts of alcohol, especially in socioeconomically disadvantaged environments, lose or give up custody of their children, who then enter the adoption system. Ninety percent of women of reproductive age in Russia consume alcohol, and 20% continue to consume it during pregnancy.16–19 A systematic review published in 2017 found a global prevalence of alcohol use in pregnancy of 9.8% and a global prevalence of FAS in the general population of 14.6/10 000, which corresponds to approximately 119 000 births per year worldwide.20 Foetal alcohol spectrum disorder is a serious public health problem given its impact at the economic, social, educational, family and health care level on affected individuals and their social circle.5–7,20
Pathophysiology of damage secondary to prenatal exposure to alcoholAlcohol toxicity depends directly on ethanol and its metabolites (acetaldehyde and reactive oxygen species [ROS]). The oxidative stress caused by alcohol and ROS is caused by an increased production of nicotinamide adenine dinucleotide (NADH), which results in an increased NAD/NADH ratio that is balanced by the formation of lactate dehydrogenase. Ethanol crosses the placenta, and while its concentration in amniotic fluid is 40% the concentration in maternal blood, its slow clearance results in prolonged foetal exposure. The foetal brain is the main target on account of its greater metabolic requirements and production of ROS combined with the lower concentration of enzymes and antioxidants.3 In addition to its teratogenic effects, alcohol exposure reduces placental perfusion, which cause ischaemia, infarction and thinning of the placenta3,5,11 that may in turn result in intrauterine growth restriction and other anatomical abnormalities. It also alters the epigenome.3,5,11,21 Although prenatal exposure is a necessary condition, the genetic aetiology and pathogenesis of the neurodevelopmental disorders observed in cases of FASD is unknown. The discovery of genetic and epigenetic markers of FASD would contribute greatly to its diagnosis.21
No amount of alcohol is safe to consume during pregnancy,5,22 although its negative effects depend mainly on the dose, duration and pattern of consumption and individual genetic susceptibility.2–4,23 Alcohol can have deleterious effects at any point in gestation, and they can all be avoided with abstinence. Discontinuing alcohol use at any point improves outcomes.6,24 Exposure often takes place during the critical period of organogenesis, when the pregnancy has not been detected yet.25 The amount of alcohol that reaches the foetus depends on the timing and volume of consumption, maternal and foetal genetics, maternal health, socioeconomic status and the family and social environment, epigenetic factors and transplacental transfer.2 The evidence on the effect of low or moderate consumption in terms of neuropsychiatric disorders in absence of organic anomalies is contradictory, as it is practically impossible to differentiate the effects of alcohol from the effects of existing confounders (tobacco and other drugs, lifestyle, dietary habits, postnatal environment, genetic factors or neglect in cases of adoption).7,15,26 The current evidence suggests that low-to-moderate prenatal exposure can result in persistent changes in multiple neurotransmitters and neuromodulators and cause neurocognitive impairment.2,25 Foetal alcohol spectrum disorder is a developmental diagnosis: it may not be immediately evident and become manifest when more complex and abstract cognitive processing and adequate social and academic functioning start to be expected, and therefore early evaluation may not reliably predict future outcomes.26,27 Significant exposure to alcohol in the first trimester is mainly associated with facial anomalies and major structural anomalies, in the second trimester, with increased risk of spontaneous miscarriage, and in the third trimester, with impaired weight gain, linear growth and brain growth. Although the clinical picture based on the timing of exposure is well defined, neurocognitive and behavioural sequelae may result from exposure at any time during gestation. Different domains may be affected: global cognition, executive function, learning, memory, language, visuospatial ability, motor function and attention as well as behaviour, with impaired adaptive skills, academic difficulties and psychiatric disorders.7,21,27,28 Most patients only exhibit impairment in areas like attention, executive function, spatial and working memory and adaptive behaviour, but a specific neurocognitive and behavioural profile for FASD has yet to be defined.2
Diagnosis of FASDThe diagnosis is based on international guidelines, with diagnostic criteria including facial features, delayed growth, structural and/or functional disorders of the central nervous system (CNS) and prenatal exposure to alcohol.2,8,21,29 The most widely used (Table 2) are the guidelines of the Institute of Medicine (IOM) of the National Academy of Sciences of the United States, the guidelines of the Centers of Disease Prevention and Control (CDC), the 4-digit diagnostic code of the University of Washington (Astley and Warren) and the Canadian guidelines.24
Comparison of international guidelines for diagnosis of FASD.
| 4-digit diagnostic code (2000) | CDC (2004) | [0,4–5]Canada (2015) | IOM updated criteria-Hoyme (2016) | ||
|---|---|---|---|---|---|
| Diagnostic term | 22 terms | FAS | FASD with sentinel facial features | FASD without sentinel facial features | FASD |
| Prenatal exposure to alcohol | Confirmed or unknown | Confirmed or unknown | Confirmed or unknown | Confirmed | Confirmed |
| Facial features | 3 of the following at any age: | 3 of the following at any age: | 3 of the following at any age: | Fewer than 3 of the following: | 2 of the following: |
| Palpebral fissure < P3 | Palpebral fissure < P3 | Palpebral fissure < P3 | Palpebral fissure < P3 | Palpebral fissure ≤ P10 | |
| Smooth philtrum, rank 4 or 5 | Smooth philtrum, rank 4 or 5 | Smooth philtrum, rank 4 or 5 | Smooth philtrum, rank 4 or 5 | Smooth philtrum, rank 4 or 5 | |
| Thin vermillion border, rank 4 or 5 | Thin vermillion border, rank 4 or 5 | Thin vermillion border, rank 4 or 5 | Thin vermillion border, rank 4 or 5 | Thin vermillion border, rank 4 or 5 | |
| Neurodevelopmental impairment | At least 1 of the following: | At least 1 of the following: | Impairment in at least 3 of the following domains: | Impairment in at least 3 of the following domains: | Impairment in 1 or 2 of the following domains: |
| 1. Structural/neurologic: e.g. HC < P3, abnormal structure, seizures, significant symptoms | 1. Structural/neurologic: e.g. HC < P10, abnormal structure, seizures, significant symptoms | Motor skills | Motor skills | Motor skills | |
| 2. Severe impairment: In 3 or more functional domains (2 or more SD below mean) | 2. Impairment: In 3 or more functional domains (1 or more SD below mean) Global deficit (2 or more SD below mean) | Neuroanatomy/neurophysiology | Neuroanatomy/neurophysiology | Neuroanatomy/neurophysiology | |
| Cognition | Cognition | Cognition | |||
| Language | Language | Language | |||
| Academic performance | Academic performance | Academic performance | |||
| Memory | Memory | Memory | |||
| Attention | Attention | Attention | |||
| Executive function, including impulse control and hyperactivity | Executive function, including impulse control and hyperactivity | Executive function, including impulse control and hyperactivity | |||
| Affect regulation | Affect regulation | Affect regulation | |||
| Adaptive regulation, social skills or social communication | Adaptive regulation, social skills or social communication | Adaptive regulation, social skills or social communication | |||
| Impaired growth | Prenatal and/or postnatal weight or height < P10 | Prenatal and/or postnatal weight or height < P10 | (not considered) | (not considered) | Prenatal and/or postnatal weight and/or height and/or HC ≤ P10 |
CDC, Centers for Disease Control and Prevention; FAS, foetal alcohol syndrome; FASD, foetal alcohol spectrum disorder; HC, head circumference; IOM, Institute of Medicine; P10, 10th percentile; P3, 3rd percentile; SD, standard deviation.
The documentation of alcohol use during pregnancy is not always easy, as it requires the collaboration of a paediatrician or geneticist and a psychologist, professionals that may not have much experience with this clinical entity. Questionnaires are not usually reliable tools, as alcohol use tends to be underestimated and underrated, possibly because consumption of low amounts is not considered dangerous or out of feelings of guilt, among other reasons. Detection of biomarkers of alcohol in biological matrices allow diagnosis of prenatal exposure.2,23–25 The detection in blood or urine indicates recent exposure. The diagnosis of alcohol use during pregnancy is based on the detection of fatty acid ethyl esters (FAEEs) or ethyl glucuronide (EtG) in placental tissue, meconium or maternal hair or nails.30 The presence of EtG in maternal hair allows retrospective identification of alcohol exposure in the entire pregnancy period (1 cm per month), and detection in meconium exposure in the second and third trimester of gestation. Using this biomarker, several studies have demonstrated that two thirds of pregnant women in Spain consume alcohol in varying amounts during gestation.10,23,25,30 Some authors have proposed a neonatal screen to detect alcohol exposure, as early intervention prevents the development of secondary disabilities and improves outcomes.23,25
There is considerable interest in identifying the neurocognitive profile of patients with FASD to guide their identification,27 as there are no universally accepted criteria and the high prevalence of psychological and psychiatric comorbidities in FASD poses challenges to accurate diagnosis. There is no known pathognomonic neurobehavioural profile or a specific pattern identified in every exposed individual, as organic damage is modulated by genetic and environmental factors.21,24 In addition, the deleterious impact of neglect and insecure attachment associated with adoption produces symptoms similar to those caused by prenatal alcohol exposure.25
A recent study attempted to identify a neurodevelopmental pattern sensitive and specific for FASD. The authors found impairments in perceptual reasoning, verbal comprehension, visual-motor speed and motor coordination, processing speed (nonverbal information), attention and executive function, visuospatial processing and language in combination with rule-breaking behaviour and attention problems. They found a neurodevelopmental and behavioural pattern that was sensitive, but not specific, for FASD, allowing differentiation of children with FASD from typically developing children but not from children with other neurodevelopmental disorders.21
Foetal alcohol spectrum syndrome is usually a diagnosis of exclusion after ruling out genetic and malformative disorders that share some of its clinical features. It is also important to take into account that women that abuse alcohol may have children with other syndromes.
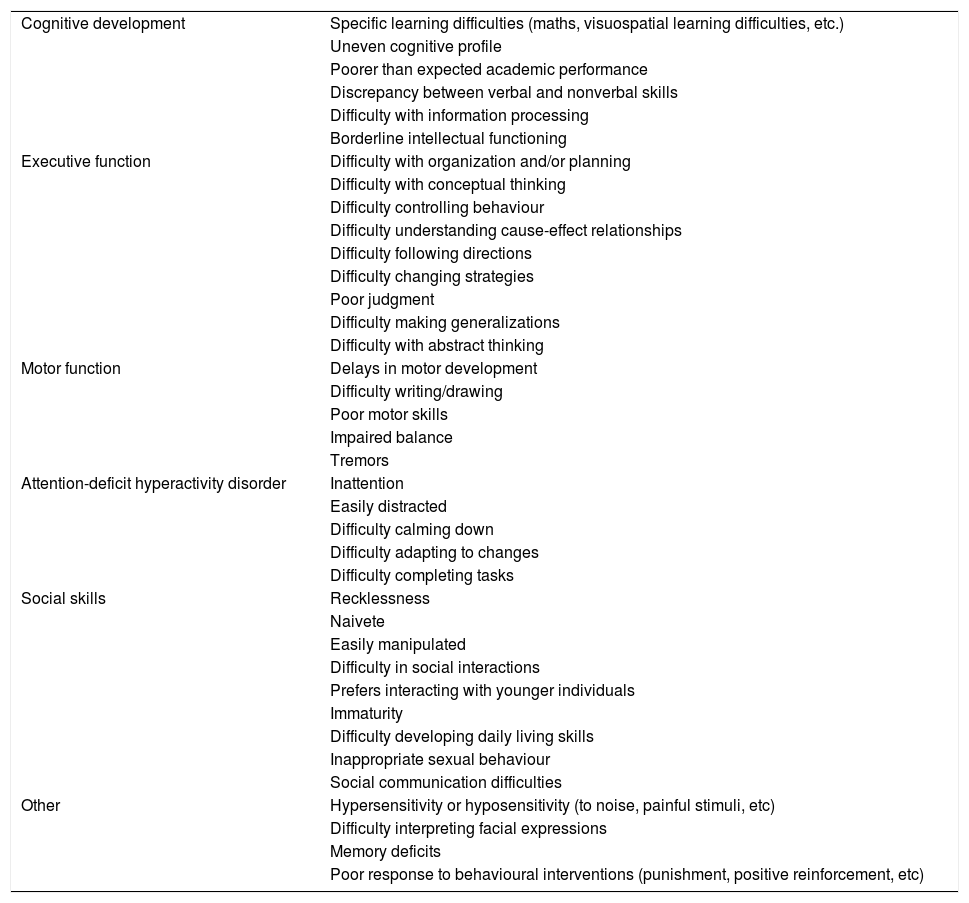
Thus, 3 areas need to be assessed that are interrelated and have to be affected: physical features; neurocognitive impairment (intellectual quotient, memory, executive function, abstract reasoning, receptive and expressive language, visual-motor integration and sensory processing, daily living and adaptive skills and processing speed) and behavioural impairment (emotional dysregulation, executive dysfunction, inattention, hyperactivity, impulsivity, irritability or negative affect, sleep disorders, and impaired social skills, adaptive behaviour and/or social communication) (Table 3). Documented consumption of alcohol during pregnancy is not a required criterion for diagnosis of FASD. It is recommended that the diagnosis of FASD is made when the patient is old enough to undergo a neuropsychological evaluation and, in case of adoption, at least 2 years after it was finalized to allow time for growth recovery and learning the language to a sufficient level for testing. The diagnosis should be made by a multidisciplinary team including psychologists, nurses, speech therapists and paediatricians.8,15,24 Foetal alcohol spectrum disorder should be considered in any child with compatible physical features and/or delayed growth or development or behavioural disorders, including ADHD or school failure, as well as children with a known history of prenatal alcohol exposure or with a sibling with FASD.6,24
Neuropsychological features of FASD.
| Cognitive development | Specific learning difficulties (maths, visuospatial learning difficulties, etc.) |
| Uneven cognitive profile | |
| Poorer than expected academic performance | |
| Discrepancy between verbal and nonverbal skills | |
| Difficulty with information processing | |
| Borderline intellectual functioning | |
| Executive function | Difficulty with organization and/or planning |
| Difficulty with conceptual thinking | |
| Difficulty controlling behaviour | |
| Difficulty understanding cause-effect relationships | |
| Difficulty following directions | |
| Difficulty changing strategies | |
| Poor judgment | |
| Difficulty making generalizations | |
| Difficulty with abstract thinking | |
| Motor function | Delays in motor development |
| Difficulty writing/drawing | |
| Poor motor skills | |
| Impaired balance | |
| Tremors | |
| Attention-deficit hyperactivity disorder | Inattention |
| Easily distracted | |
| Difficulty calming down | |
| Difficulty adapting to changes | |
| Difficulty completing tasks | |
| Social skills | Recklessness |
| Naivete | |
| Easily manipulated | |
| Difficulty in social interactions | |
| Prefers interacting with younger individuals | |
| Immaturity | |
| Difficulty developing daily living skills | |
| Inappropriate sexual behaviour | |
| Social communication difficulties | |
| Other | Hypersensitivity or hyposensitivity (to noise, painful stimuli, etc) |
| Difficulty interpreting facial expressions | |
| Memory deficits | |
| Poor response to behavioural interventions (punishment, positive reinforcement, etc) |
FASD, foetal alcohol spectrum disorder.
Phenotypic diagnostic criteria (dysmorphic features and abnormal growth patterns) are easy to identify, but the neurocognitive and behavioural features of FASD are shared by other neurodevelopmental and psychiatric disorders: attention-deficit hyperactivity disorder (ADHD), autism spectrum disorder (ASD), global developmental delay, intellectual disability, oppositional-defiant disorder, conduct disorder, mood disorders (depression, bipolar disorder), reactive attachment disorder, post-traumatic stress, sleep disturbances, substance use and schizophrenia.
Landgren et al. assessed the presence of neurodevelopmental disorders in children adopted from Eastern Europe in Sweden 5 years after adoption. They found FASD in 52% (30% FAS, 14% partial FAS and 9% ARND) and ADHD in 51%. In addition, 23% had intellectual disability or significant cognitive impairment, 9% autism and 34% developmental coordination disorder.13 During the post-adoption follow-up, 46% developed neurodevelopmental or psychiatric disorders: 8% ASD, 24% attention and behavioural disorders, 21% cognitive impairment, 11% language disorder, 4% epilepsy and 3% cerebral palsy. A diagnosis of FAS is made or considered in 5% of cases.13,15,18 A study conducted in Catalonia in children adopted from Eastern European countries found that 50% had FASD.14 Environmental factors and/or factors related to the personal history (adversity, trauma, early loss, intrauterine exposure to toxic substances, poverty, disadvantaged environment, abuse and/or neglect) can contribute to a neuropsychological profile similar to that of FASD. All these factors can affect areas of the brain involved in executive function, memory, affect regulation, attention and the response to stress. It is not always possible to determine the extent of the contribution of prenatal alcohol exposure versus other environmental factors. Institutionalization in an orphanage or a similar facility is a risk factor for neuropsychiatric morbidity, so the presence of neurocognitive or psychiatric disorders (diagnostic criteria for FASD) may not be explained exclusively by alcohol exposure.2,21,31,32
International adoption and neurocognitive disordersBetween 2014 and 2018, there were 3174 adoptions in Spain. The most frequent region of origin was Asia (50.8%), followed by Eastern Europe (23.3%).33 The prevalence of FASD in children adopted from these regions is substantial (50%).15,20,34,35 Neuropsychological problems are more frequent in these infants compared to infants from other regions, with a higher prevalence of attention problems, learning difficulties, hyperactivity, difficulties in interpersonal relationships and insecure attachment. A dual pathogenesis has been proposed in these children: prenatal exposure to alcohol and neglect/institutionalization.25 Insecure attachment leads to difficulty handling changes in routines, moving from one task to another and adjusting to the local community and school environments, interpersonal difficulties with parents and peers, hyperactivity and inattention.21,33 A study in families with internationally adopted children found that children from Eastern Europe are more likely to exhibit behavioural disorders, anger, interpersonal difficulties and ADHD with or without hyperactivity and to require prescription medication compared to children adopted from other regions. These families are also more likely to seek mental health services.25,35,36
Institutionalised children may have experienced deficiencies in the prenatal and postnatal periods. Factors other than prenatal alcohol exposure may be at play. Variants in genes involved in glycosylation could be associated with more severe clinical forms.37 The consumption of alcohol harms the foetus directly but also indirectly through its association with maternal malnutrition. There are different factors involved in this association, such as socioeconomic status in relation to the consumption of alcohol or other drugs, the additional calories contributed by alcohol to the total energy intake, the nutrient malabsorption secondary to chronic alcohol use and the interactions of alcohol metabolites with different essential amino acids and vitamins (thiamine, riboflavin, pyridoxine, vitamin A, vitamin C and folic acid).38 Peri- and postnatal deficiencies associated with institutionalization include malnutrition, micronutrient deficiencies, understimulation or physical, emotional or environmental deprivation, infectious diseases and exposure to environmental neurotoxins. Perinatal complications that may result in increased clinical severity are also frequent, including low birth weight, preterm birth, lack of prenatal care, exposure to drugs and alcohol and stress, depression or other psychiatric disorders in the mother.17 On the other hand, the age at the time of adoption determines the time that the infant remains exposed to these harmful factors. Sequelae largely depend on whether the neural pathways involved in plasticity and reorganization are preserved at the time the child moves into a stimulating environment.39 Understimulation during the critical period of maximum developmental plasticity (the first 3 years of life) can have short- and long-term repercussions on physical and mental health outcomes. There is evidence that adoption after age 18 months is associated with a drastic increase in social disability.40
Treatment of FASDThe damage caused during intrauterine life by prenatal alcohol exposure is irreversible.26 Nevertheless, early intervention can prevent the development of secondary disorders and improve neurodevelopmental outcomes.2,25 Treatment is mainly neuropsychiatric and based on optimising the household, school and work environments through behavioural and/or educational interventions with the aim of improving long-term outcomes in language, reading, social and organizational skills, psychomotor development and autonomy, among other domains.21,26,31,32,40 Recommendations for management include establishing a safe, stable and structured household environment with predictable routines and daily activities, setting realistic goals and expectations, providing an appropriate and adaptable school environment with limited environmental stimuli; promoting skill development; giving simple and specific directions; teaching clear and immediate consequences of behaviours; using multisensory instruction techniques (visual, auditory, with a hands-on approach) and teaching how to identify difficulties and ask for help, anger management skills, social skills or techniques to control arousal.5,27
Several studies have focused on the treatment of nutritional deficiencies secondary to alcohol use and eating and social disorders in children with FASD, although few propose the use of postnatal nutritional therapy for management of neurodevelopmental disorders. Different nutrients are currently being investigated (vitamin A, docosahexaenoic acid, resveratrol, choline and epigallocatechin gallate).11,38
Another treatment option that is currently under investigation is the administration of antioxidants to reverse the intracellular redox imbalance. Evidence from animal models shows that vitamin C, a synthetic complex similar to superoxide dismutase and catalase (EUK-134), vitamin E, black or Panax ginseng and anthocyanins like C3G or cyanidin-3-glucoside can prevent or reduce growth delay and development of malformations following ethanol exposure and have effects at the neuroanatomical level. The evidence on the role of antioxidants for management of behavioural disorders is inconclusive. Improvements in behaviour and learning have been observed in some cases. Antioxidants, alone or combined with other drugs, may mitigate some of the observed disorders, although further research is required to determine the optimal combination therapy.3
Stimulants are widely used to reduce symptoms of ADHD, although there is little evidence of their use in the context of FASD. It seems that methylphenidate and lisdexamfetamine can reduce hyperactivity and impulsivity, with lesser effects on inattention. The effects of atomoxetine for management of FAS is currently being investigated, as it may be useful in cases with associated ADHD or anxiety/tic disorders.29 Some patients require a combination of stimulant and nonstimulant medication, or possibly and antipsychotic (mainly risperidone) in case of behavioural disorders associated with ADHD refractory to other treatments. Children managed with antipsychotics achieve better outcomes.6,40
ConclusionsIn many cases, the diagnosis of FASD is delayed or entirely missed because its presentation overlaps with the behavioural or cognitive features attributable to other aetiologies also associated with prenatal alcohol exposure, such as neglect or attachment problems. A neurodevelopmental profile (neurocognitive and behavioural) pathognomonic for FASD has not been established to date, although this aspect is starting to be explored in the scientific literature.21 Hopefully, the eventual identification of a specific profile combining neurodevelopmental features and genetic and epigenetic data will allow diagnosis of FASD and discrimination between FASD, similar disorders and morbidity secondary to neglect.
FundingThe study did not receive any form of funding.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Maya-Enero S, Ramis-Fernández SM, Astals-Vizcaino M, García-Algar Ó. Perfil neurocognitvo y conductual del trastorno del espectro alcohólico fetal. An Pediatr (Barc). 2021;95:208.