We present the summary of a critical appraisal document of the available evidence on COVID-19, developed with a clinical practice guide format following GRADE methodology. The document tries to provide answers to a series of structured clinical questions, with an explicit definition of the population, intervention / exposure, comparison and outcome, and a rating of the clinical relevance of the outcome measures. We conducted a systematic review of the literature to answer the questions, grouped into six chapters: epidemiology, clinical practice, diagnosis, treatment, prevention, and vaccination. We assessed the risk of bias of the selected studies with standard instruments (RoB-2, ROBINS-I, QUADAS and Newcastle-Ottawa). We constructed evidence tables and, when necessary and possible, meta-analysis of the of the most relevant outcome measures. We followed the GRADE system to synthesise the evidence, assessing its quality, and, when appropriate, giving recommendations, rated according to the quality of the evidence, the values and preferences, the balance between benefits, risks and costs, equity and feasibility.
Presentamos el resumen de un documento de valoración crítica de la evidencia disponible sobre COVID-19, elaborado con formato de guía de práctica clínica siguiendo metodología GRADE. El documento trata de dar respuestas a una serie de preguntas clínicas estructuradas, con definición explícita de la población, intervención/exposición, comparación y resultado, y una jerarquización de la importancia clínica de las medidas de efecto valoradas. Realizamos revisiones sistemáticas de la literatura para responder a las preguntas, agrupadas en seis capítulos: epidemiología, clínica, diagnóstico, tratamiento, prevención y vacunas. Valoramos el riesgo de sesgo de los estudios seleccionados con instrumentos estándar (RoB-2, ROBINS-I, QUADAS y Newcastle-Ottawa). Elaboramos tablas de evidencia y, cuando fue necesario y posible, metanálisis de las principales medidas de efecto. Seguimos el sistema GRADE para realizar síntesis de la evidencia, con valoración de su calidad, y, cuando se consideró apropiado, emitir recomendaciones, jerarquizadas en función de la calidad de la evidencia, los valores y preferencias, el balance entre beneficios, riesgos y costes, la equidad y la factibilidad.
The pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is having an enormous impact worldwide, and especially in Spain. It poses a challenge to our health care system, with important health, social and economic repercussions due to the impact on the way of life that results from the fight against the disease and the associated morbidity and mortality.
There is a growing body of evidence on coronavirus disease 2019 (COVID-19), with initial research conducted on an urgent basis and under exceptional circumstances, which led to the publication of studies with significant limitations. The accrued evidence has acquired an astonishing magnitude in its volume, immediacy and, increasingly, its quality and collaborative nature. The analysis of the research published to date is not only a challenge in terms of quantity, but also in terms of quality, as it requires identifying which studies are valid and which results applicable.
The paediatric population has not been as affected as other age groups, as most infections in this group have been mild or asymptomatic, with few patients developing potentially severe forms of disease. Consequently, a large part of the available data, from both observational and, especially, experimental studies, has focused on the adult population. This is the body of evidence in which we have to search for answers to most of the questions paediatricians have about the infection.
Given the circumstances, the Committee on Evidence-Based Paediatrics of the Asociación Española de Pediatría (Spanish Association of Pediatrics, AEP) and the Asociación Española de Pediatría de Atención Primaria (Spanish Association of Primary Care Paediatrics, AEPap) felt the need to develop a document on the current evidence on the subject of COVID-19 in the paediatric population (0−18 years), addressing every aspect of the disease: epidemiology, clinical characteristics, diagnosis, treatment, prevention and vaccination.
To search, evaluate, synthesise and establish the relative importance of the available evidence and to develop recommendations based on this evidence is a challenge: it requires that we navigate uncertainty and handle a substantial amount of information, in large part indirect. Our aim was to offer a summary of the evidence published to date accompanied by an assessment of its quality, information that must provide the foundation for clinical decision-making, adapted to the circumstances of each patient. Our intent was not to provide directions to be applied as presented, and this document should not be interpreted as a protocol; instead, we aimed to provide information with which to form the necessary judgments to optimise the management of paediatric patients. This information must be combined with clinical experience and expert opinion to develop the protocols demanded by our health care system and society at large.
MethodsTo develop this document, we have adhered in part to the methodology manual for the development of clinical practice guidelines of the public health system of Spain.1 We assessed the quality of the evidence and established the strength of recommendations applying the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach.2 The full document provides a detailed description of the methods used.3
In short, the steps were as follows: 1) formulation of structured clinical questions to address 2) performance of systematic literature searches, with updates on an ongoing basis; 3) selection and evaluation of studies, assessing the risk of bias (with the RoB2, ROBINS-I, QUADAS and Newcastle-Ottawa instruments); 4) qualitative and/or quantitative synthesis (meta-analysis) of the main outcome measures; 5) classification of the quality of the evidence available for each clinical question, which entailed issuing an overall rating for the quality of the data on the outcomes relevant to the question, and 6) development of recommendations, if the evidence allowed, taking into account the GRADE criteria (quality of evidence, values and preferences, balance of benefits, harms and risks, equity and feasibility).2
Results and discussionWe present the summary of the available evidence and related recommendations in tables focused on epidemiology (Table 1), clinical characteristics (Table 2), diagnostic tests (Table 3), treatment (Table 4), prevention (Table 5) and vaccination (Table 6).
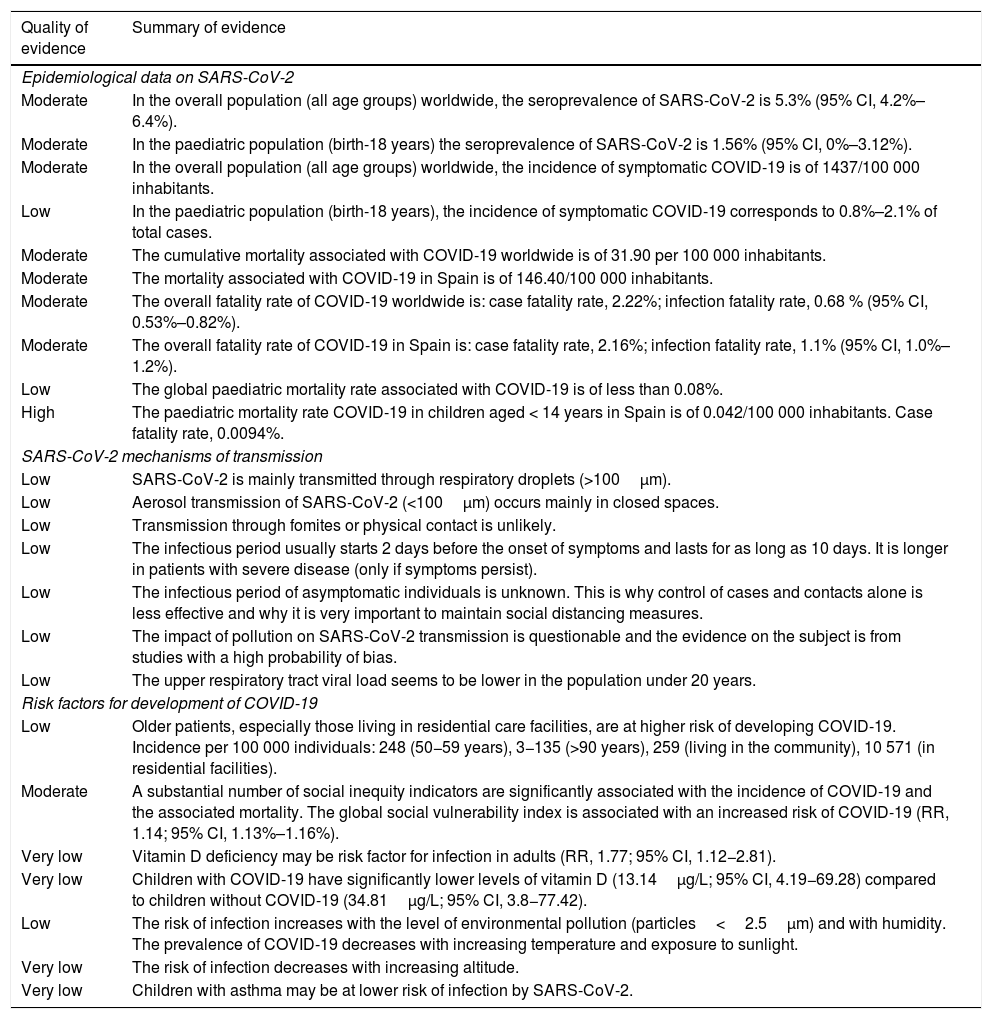
Epidemiology of COVID-19 in the paediatric population, mechanisms of transmission of SARS-CoV-2 and risk factors for COVID-19.
| Quality of evidence | Summary of evidence |
|---|---|
| Epidemiological data on SARS-CoV-2 | |
| Moderate | In the overall population (all age groups) worldwide, the seroprevalence of SARS-CoV-2 is 5.3% (95% CI, 4.2%–6.4%). |
| Moderate | In the paediatric population (birth-18 years) the seroprevalence of SARS-CoV-2 is 1.56% (95% CI, 0%–3.12%). |
| Moderate | In the overall population (all age groups) worldwide, the incidence of symptomatic COVID-19 is of 1437/100 000 inhabitants. |
| Low | In the paediatric population (birth-18 years), the incidence of symptomatic COVID-19 corresponds to 0.8%–2.1% of total cases. |
| Moderate | The cumulative mortality associated with COVID-19 worldwide is of 31.90 per 100 000 inhabitants. |
| Moderate | The mortality associated with COVID-19 in Spain is of 146.40/100 000 inhabitants. |
| Moderate | The overall fatality rate of COVID-19 worldwide is: case fatality rate, 2.22%; infection fatality rate, 0.68 % (95% CI, 0.53%–0.82%). |
| Moderate | The overall fatality rate of COVID-19 in Spain is: case fatality rate, 2.16%; infection fatality rate, 1.1% (95% CI, 1.0%–1.2%). |
| Low | The global paediatric mortality rate associated with COVID-19 is of less than 0.08%. |
| High | The paediatric mortality rate COVID-19 in children aged < 14 years in Spain is of 0.042/100 000 inhabitants. Case fatality rate, 0.0094%. |
| SARS-CoV-2 mechanisms of transmission | |
| Low | SARS-CoV-2 is mainly transmitted through respiratory droplets (>100μm). |
| Low | Aerosol transmission of SARS-CoV-2 (<100μm) occurs mainly in closed spaces. |
| Low | Transmission through fomites or physical contact is unlikely. |
| Low | The infectious period usually starts 2 days before the onset of symptoms and lasts for as long as 10 days. It is longer in patients with severe disease (only if symptoms persist). |
| Low | The infectious period of asymptomatic individuals is unknown. This is why control of cases and contacts alone is less effective and why it is very important to maintain social distancing measures. |
| Low | The impact of pollution on SARS-CoV-2 transmission is questionable and the evidence on the subject is from studies with a high probability of bias. |
| Low | The upper respiratory tract viral load seems to be lower in the population under 20 years. |
| Risk factors for development of COVID-19 | |
| Low | Older patients, especially those living in residential care facilities, are at higher risk of developing COVID-19. Incidence per 100 000 individuals: 248 (50−59 years), 3−135 (>90 years), 259 (living in the community), 10 571 (in residential facilities). |
| Moderate | A substantial number of social inequity indicators are significantly associated with the incidence of COVID-19 and the associated mortality. The global social vulnerability index is associated with an increased risk of COVID-19 (RR, 1.14; 95% CI, 1.13%–1.16%). |
| Very low | Vitamin D deficiency may be risk factor for infection in adults (RR, 1.77; 95% CI, 1.12−2.81). |
| Very low | Children with COVID-19 have significantly lower levels of vitamin D (13.14μg/L; 95% CI, 4.19−69.28) compared to children without COVID-19 (34.81μg/L; 95% CI, 3.8−77.42). |
| Low | The risk of infection increases with the level of environmental pollution (particles<2.5μm) and with humidity. The prevalence of COVID-19 decreases with increasing temperature and exposure to sunlight. |
| Very low | The risk of infection decreases with increasing altitude. |
| Very low | Children with asthma may be at lower risk of infection by SARS-CoV-2. |
CI, confidence interval; RR, relative risk.
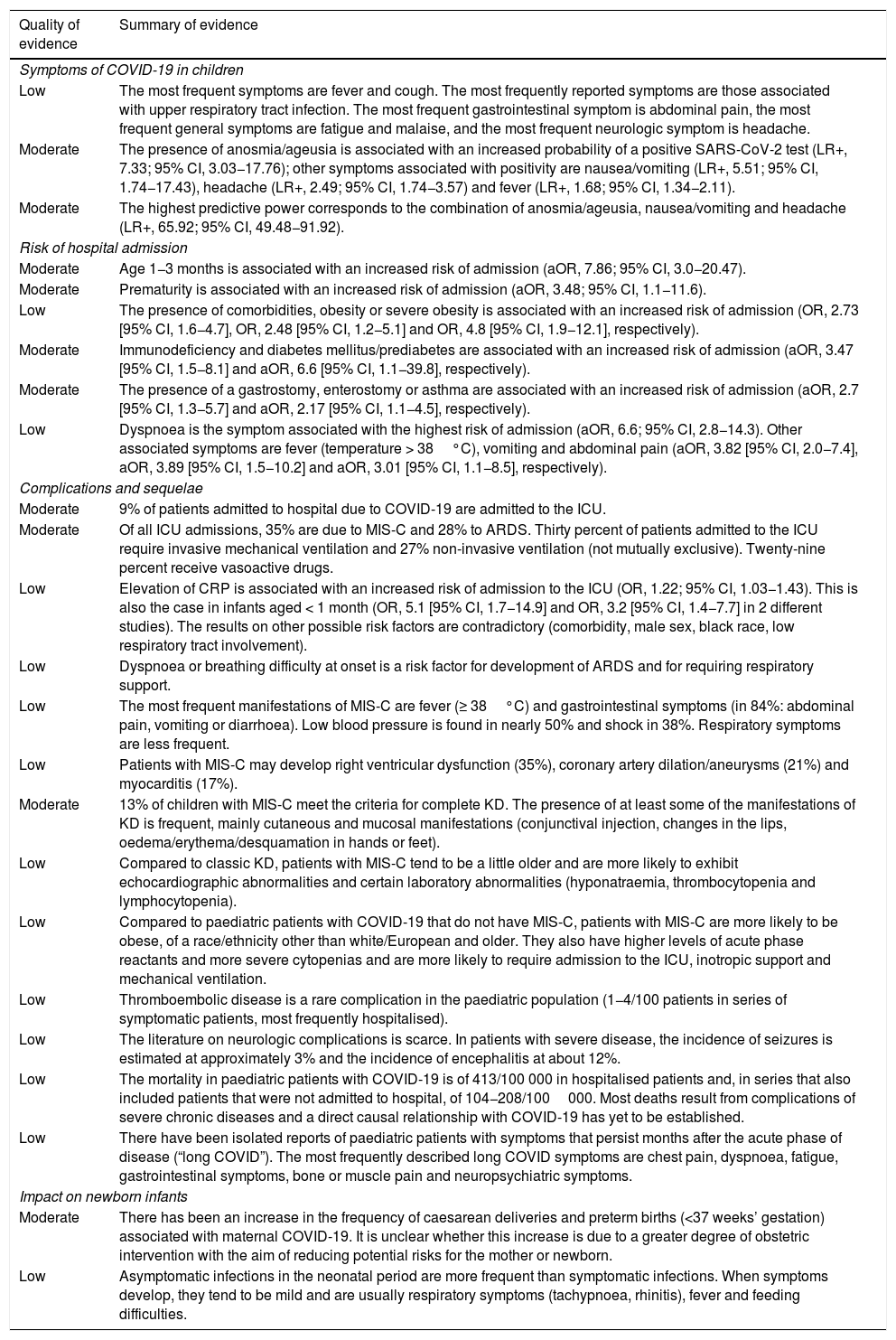
Summary and quality of the evidence on the manifestations of COVID-19 in the paediatric population.
| Quality of evidence | Summary of evidence |
|---|---|
| Symptoms of COVID-19 in children | |
| Low | The most frequent symptoms are fever and cough. The most frequently reported symptoms are those associated with upper respiratory tract infection. The most frequent gastrointestinal symptom is abdominal pain, the most frequent general symptoms are fatigue and malaise, and the most frequent neurologic symptom is headache. |
| Moderate | The presence of anosmia/ageusia is associated with an increased probability of a positive SARS-CoV-2 test (LR+, 7.33; 95% CI, 3.03−17.76); other symptoms associated with positivity are nausea/vomiting (LR+, 5.51; 95% CI, 1.74−17.43), headache (LR+, 2.49; 95% CI, 1.74−3.57) and fever (LR+, 1.68; 95% CI, 1.34−2.11). |
| Moderate | The highest predictive power corresponds to the combination of anosmia/ageusia, nausea/vomiting and headache (LR+, 65.92; 95% CI, 49.48−91.92). |
| Risk of hospital admission | |
| Moderate | Age 1−3 months is associated with an increased risk of admission (aOR, 7.86; 95% CI, 3.0−20.47). |
| Moderate | Prematurity is associated with an increased risk of admission (aOR, 3.48; 95% CI, 1.1−11.6). |
| Low | The presence of comorbidities, obesity or severe obesity is associated with an increased risk of admission (OR, 2.73 [95% CI, 1.6−4.7], OR, 2.48 [95% CI, 1.2−5.1] and OR, 4.8 [95% CI, 1.9−12.1], respectively). |
| Moderate | Immunodeficiency and diabetes mellitus/prediabetes are associated with an increased risk of admission (aOR, 3.47 [95% CI, 1.5−8.1] and aOR, 6.6 [95% CI, 1.1−39.8], respectively). |
| Moderate | The presence of a gastrostomy, enterostomy or asthma are associated with an increased risk of admission (aOR, 2.7 [95% CI, 1.3−5.7] and aOR, 2.17 [95% CI, 1.1−4.5], respectively). |
| Low | Dyspnoea is the symptom associated with the highest risk of admission (aOR, 6.6; 95% CI, 2.8−14.3). Other associated symptoms are fever (temperature > 38°C), vomiting and abdominal pain (aOR, 3.82 [95% CI, 2.0−7.4], aOR, 3.89 [95% CI, 1.5−10.2] and aOR, 3.01 [95% CI, 1.1−8.5], respectively). |
| Complications and sequelae | |
| Moderate | 9% of patients admitted to hospital due to COVID-19 are admitted to the ICU. |
| Moderate | Of all ICU admissions, 35% are due to MIS-C and 28% to ARDS. Thirty percent of patients admitted to the ICU require invasive mechanical ventilation and 27% non-invasive ventilation (not mutually exclusive). Twenty-nine percent receive vasoactive drugs. |
| Low | Elevation of CRP is associated with an increased risk of admission to the ICU (OR, 1.22; 95% CI, 1.03−1.43). This is also the case in infants aged < 1 month (OR, 5.1 [95% CI, 1.7−14.9] and OR, 3.2 [95% CI, 1.4−7.7] in 2 different studies). The results on other possible risk factors are contradictory (comorbidity, male sex, black race, low respiratory tract involvement). |
| Low | Dyspnoea or breathing difficulty at onset is a risk factor for development of ARDS and for requiring respiratory support. |
| Low | The most frequent manifestations of MIS-C are fever (≥ 38°C) and gastrointestinal symptoms (in 84%: abdominal pain, vomiting or diarrhoea). Low blood pressure is found in nearly 50% and shock in 38%. Respiratory symptoms are less frequent. |
| Low | Patients with MIS-C may develop right ventricular dysfunction (35%), coronary artery dilation/aneurysms (21%) and myocarditis (17%). |
| Moderate | 13% of children with MIS-C meet the criteria for complete KD. The presence of at least some of the manifestations of KD is frequent, mainly cutaneous and mucosal manifestations (conjunctival injection, changes in the lips, oedema/erythema/desquamation in hands or feet). |
| Low | Compared to classic KD, patients with MIS-C tend to be a little older and are more likely to exhibit echocardiographic abnormalities and certain laboratory abnormalities (hyponatraemia, thrombocytopenia and lymphocytopenia). |
| Low | Compared to paediatric patients with COVID-19 that do not have MIS-C, patients with MIS-C are more likely to be obese, of a race/ethnicity other than white/European and older. They also have higher levels of acute phase reactants and more severe cytopenias and are more likely to require admission to the ICU, inotropic support and mechanical ventilation. |
| Low | Thromboembolic disease is a rare complication in the paediatric population (1−4/100 patients in series of symptomatic patients, most frequently hospitalised). |
| Low | The literature on neurologic complications is scarce. In patients with severe disease, the incidence of seizures is estimated at approximately 3% and the incidence of encephalitis at about 12%. |
| Low | The mortality in paediatric patients with COVID-19 is of 413/100 000 in hospitalised patients and, in series that also included patients that were not admitted to hospital, of 104−208/100000. Most deaths result from complications of severe chronic diseases and a direct causal relationship with COVID-19 has yet to be established. |
| Low | There have been isolated reports of paediatric patients with symptoms that persist months after the acute phase of disease (“long COVID”). The most frequently described long COVID symptoms are chest pain, dyspnoea, fatigue, gastrointestinal symptoms, bone or muscle pain and neuropsychiatric symptoms. |
| Impact on newborn infants | |
| Moderate | There has been an increase in the frequency of caesarean deliveries and preterm births (<37 weeks’ gestation) associated with maternal COVID-19. It is unclear whether this increase is due to a greater degree of obstetric intervention with the aim of reducing potential risks for the mother or newborn. |
| Low | Asymptomatic infections in the neonatal period are more frequent than symptomatic infections. When symptoms develop, they tend to be mild and are usually respiratory symptoms (tachypnoea, rhinitis), fever and feeding difficulties. |
aOR, adjusted odds ratio; ARDS, acute respiratory distress syndrome; CRP, C-reactive protein; ICU, intensive care unit; LR+, positive likelihood ratio; KD, Kawasaki disease; MIS-C, multisystemic inflammatory syndrome in children associated with SARS-CoV-2.
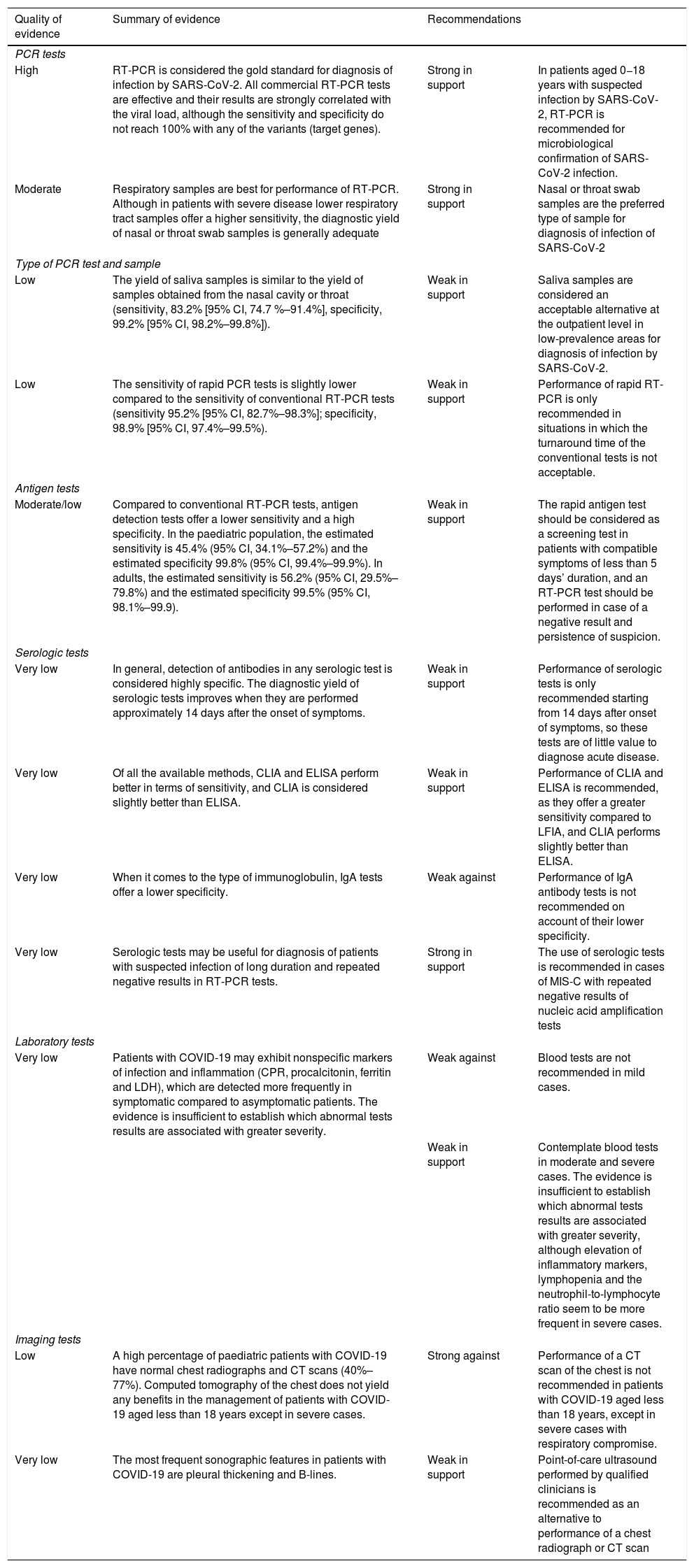
Summary and quality of the evidence and recommendations on the tests used for diagnosis of COVID-19 in the paediatric population.
| Quality of evidence | Summary of evidence | Recommendations | |
|---|---|---|---|
| PCR tests | |||
| High | RT-PCR is considered the gold standard for diagnosis of infection by SARS-CoV-2. All commercial RT-PCR tests are effective and their results are strongly correlated with the viral load, although the sensitivity and specificity do not reach 100% with any of the variants (target genes). | Strong in support | In patients aged 0−18 years with suspected infection by SARS-CoV-2, RT-PCR is recommended for microbiological confirmation of SARS-CoV-2 infection. |
| Moderate | Respiratory samples are best for performance of RT-PCR. Although in patients with severe disease lower respiratory tract samples offer a higher sensitivity, the diagnostic yield of nasal or throat swab samples is generally adequate | Strong in support | Nasal or throat swab samples are the preferred type of sample for diagnosis of infection of SARS-CoV-2 |
| Type of PCR test and sample | |||
| Low | The yield of saliva samples is similar to the yield of samples obtained from the nasal cavity or throat (sensitivity, 83.2% [95% CI, 74.7 %–91.4%], specificity, 99.2% [95% CI, 98.2%–99.8%]). | Weak in support | Saliva samples are considered an acceptable alternative at the outpatient level in low-prevalence areas for diagnosis of infection by SARS-CoV-2. |
| Low | The sensitivity of rapid PCR tests is slightly lower compared to the sensitivity of conventional RT-PCR tests (sensitivity 95.2% [95% CI, 82.7%–98.3%]; specificity, 98.9% [95% CI, 97.4%–99.5%). | Weak in support | Performance of rapid RT-PCR is only recommended in situations in which the turnaround time of the conventional tests is not acceptable. |
| Antigen tests | |||
| Moderate/low | Compared to conventional RT-PCR tests, antigen detection tests offer a lower sensitivity and a high specificity. In the paediatric population, the estimated sensitivity is 45.4% (95% CI, 34.1%–57.2%) and the estimated specificity 99.8% (95% CI, 99.4%–99.9%). In adults, the estimated sensitivity is 56.2% (95% CI, 29.5%–79.8%) and the estimated specificity 99.5% (95% CI, 98.1%–99.9). | Weak in support | The rapid antigen test should be considered as a screening test in patients with compatible symptoms of less than 5 days’ duration, and an RT-PCR test should be performed in case of a negative result and persistence of suspicion. |
| Serologic tests | |||
| Very low | In general, detection of antibodies in any serologic test is considered highly specific. The diagnostic yield of serologic tests improves when they are performed approximately 14 days after the onset of symptoms. | Weak in support | Performance of serologic tests is only recommended starting from 14 days after onset of symptoms, so these tests are of little value to diagnose acute disease. |
| Very low | Of all the available methods, CLIA and ELISA perform better in terms of sensitivity, and CLIA is considered slightly better than ELISA. | Weak in support | Performance of CLIA and ELISA is recommended, as they offer a greater sensitivity compared to LFIA, and CLIA performs slightly better than ELISA. |
| Very low | When it comes to the type of immunoglobulin, IgA tests offer a lower specificity. | Weak against | Performance of IgA antibody tests is not recommended on account of their lower specificity. |
| Very low | Serologic tests may be useful for diagnosis of patients with suspected infection of long duration and repeated negative results in RT-PCR tests. | Strong in support | The use of serologic tests is recommended in cases of MIS-C with repeated negative results of nucleic acid amplification tests |
| Laboratory tests | |||
| Very low | Patients with COVID-19 may exhibit nonspecific markers of infection and inflammation (CPR, procalcitonin, ferritin and LDH), which are detected more frequently in symptomatic compared to asymptomatic patients. The evidence is insufficient to establish which abnormal tests results are associated with greater severity. | Weak against | Blood tests are not recommended in mild cases. |
| Weak in support | Contemplate blood tests in moderate and severe cases. The evidence is insufficient to establish which abnormal tests results are associated with greater severity, although elevation of inflammatory markers, lymphopenia and the neutrophil-to-lymphocyte ratio seem to be more frequent in severe cases. | ||
| Imaging tests | |||
| Low | A high percentage of paediatric patients with COVID-19 have normal chest radiographs and CT scans (40%–77%). Computed tomography of the chest does not yield any benefits in the management of patients with COVID-19 aged less than 18 years except in severe cases. | Strong against | Performance of a CT scan of the chest is not recommended in patients with COVID-19 aged less than 18 years, except in severe cases with respiratory compromise. |
| Very low | The most frequent sonographic features in patients with COVID-19 are pleural thickening and B-lines. | Weak in support | Point-of-care ultrasound performed by qualified clinicians is recommended as an alternative to performance of a chest radiograph or CT scan |
CI, confidence interval; CLIA, chemiluminescence immunoassay; CPR, C-reactive protein; CT, computed tomography; ELISA, enzyme linked immunosorbent assay; IgA, immunoglobulin A; LDH, lactate dehydrogenase; LFIA, lateral flow immunoassay; MIS-C, multisystem inflammatory syndrome in children linked to SARS-CoV-2; PCR, polymerase chain reaction; RT-PCR, reverse transcription polymerase chain reaction.
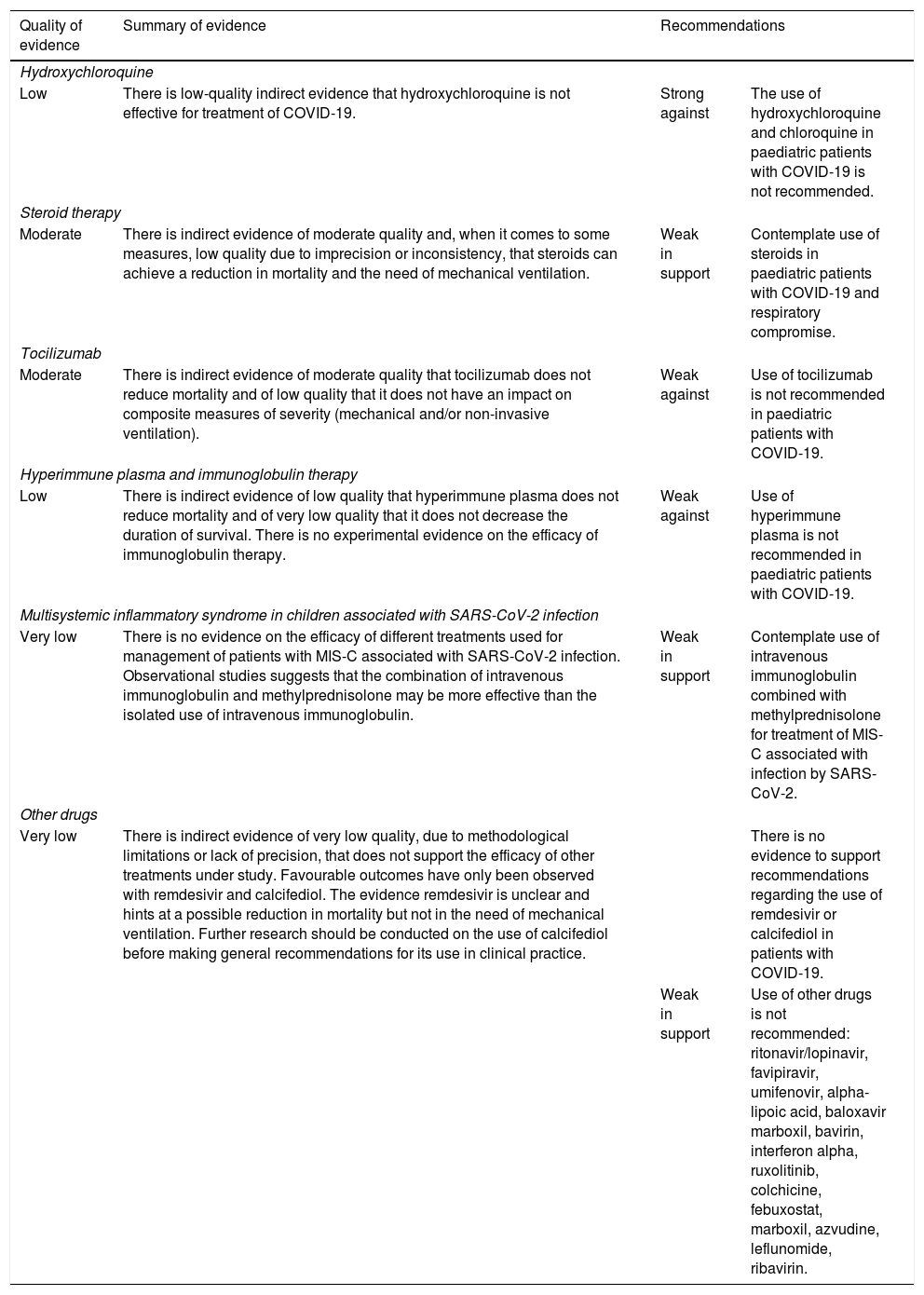
Summary and quality of the evidence and recommendations on the pharmacological treatment of COVID-19.
| Quality of evidence | Summary of evidence | Recommendations | |
|---|---|---|---|
| Hydroxychloroquine | |||
| Low | There is low-quality indirect evidence that hydroxychloroquine is not effective for treatment of COVID-19. | Strong against | The use of hydroxychloroquine and chloroquine in paediatric patients with COVID-19 is not recommended. |
| Steroid therapy | |||
| Moderate | There is indirect evidence of moderate quality and, when it comes to some measures, low quality due to imprecision or inconsistency, that steroids can achieve a reduction in mortality and the need of mechanical ventilation. | Weak in support | Contemplate use of steroids in paediatric patients with COVID-19 and respiratory compromise. |
| Tocilizumab | |||
| Moderate | There is indirect evidence of moderate quality that tocilizumab does not reduce mortality and of low quality that it does not have an impact on composite measures of severity (mechanical and/or non-invasive ventilation). | Weak against | Use of tocilizumab is not recommended in paediatric patients with COVID-19. |
| Hyperimmune plasma and immunoglobulin therapy | |||
| Low | There is indirect evidence of low quality that hyperimmune plasma does not reduce mortality and of very low quality that it does not decrease the duration of survival. There is no experimental evidence on the efficacy of immunoglobulin therapy. | Weak against | Use of hyperimmune plasma is not recommended in paediatric patients with COVID-19. |
| Multisystemic inflammatory syndrome in children associated with SARS-CoV-2 infection | |||
| Very low | There is no evidence on the efficacy of different treatments used for management of patients with MIS-C associated with SARS-CoV-2 infection. Observational studies suggests that the combination of intravenous immunoglobulin and methylprednisolone may be more effective than the isolated use of intravenous immunoglobulin. | Weak in support | Contemplate use of intravenous immunoglobulin combined with methylprednisolone for treatment of MIS-C associated with infection by SARS-CoV-2. |
| Other drugs | |||
| Very low | There is indirect evidence of very low quality, due to methodological limitations or lack of precision, that does not support the efficacy of other treatments under study. Favourable outcomes have only been observed with remdesivir and calcifediol. The evidence remdesivir is unclear and hints at a possible reduction in mortality but not in the need of mechanical ventilation. Further research should be conducted on the use of calcifediol before making general recommendations for its use in clinical practice. | There is no evidence to support recommendations regarding the use of remdesivir or calcifediol in patients with COVID-19. | |
| Weak in support | Use of other drugs is not recommended: ritonavir/lopinavir, favipiravir, umifenovir, alpha-lipoic acid, baloxavir marboxil, bavirin, interferon alpha, ruxolitinib, colchicine, febuxostat, marboxil, azvudine, leflunomide, ribavirin. | ||
MIS-C, multisystemic inflammatory syndrome in children associated with SARS-CoV-2.
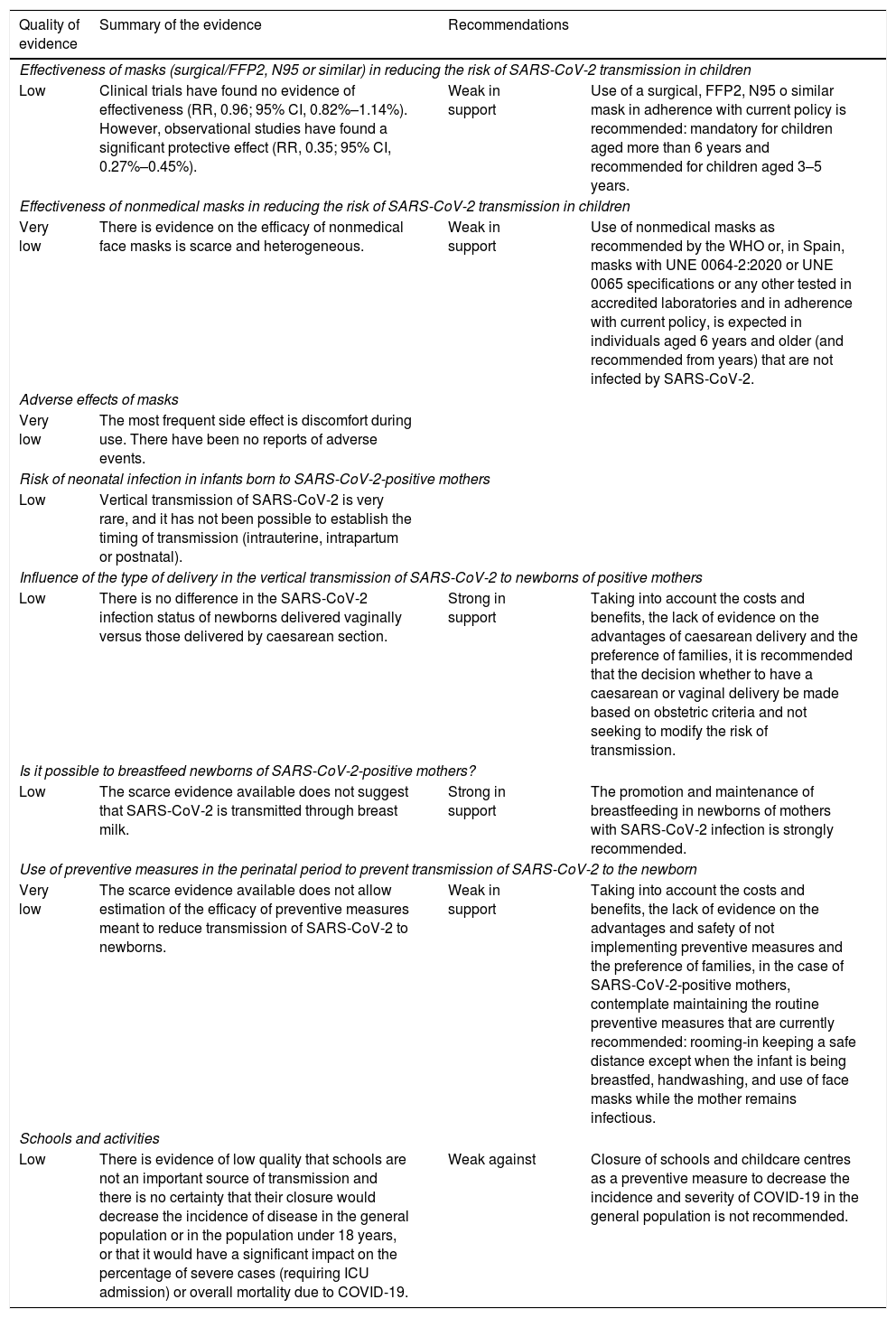
Summary and quality of the evidence and recommendations on the prevention of COVID-19 in the paediatric population.
| Quality of evidence | Summary of the evidence | Recommendations | |
|---|---|---|---|
| Effectiveness of masks (surgical/FFP2, N95 or similar) in reducing the risk of SARS-CoV-2 transmission in children | |||
| Low | Clinical trials have found no evidence of effectiveness (RR, 0.96; 95% CI, 0.82%–1.14%). However, observational studies have found a significant protective effect (RR, 0.35; 95% CI, 0.27%–0.45%). | Weak in support | Use of a surgical, FFP2, N95 o similar mask in adherence with current policy is recommended: mandatory for children aged more than 6 years and recommended for children aged 3–5 years. |
| Effectiveness of nonmedical masks in reducing the risk of SARS-CoV-2 transmission in children | |||
| Very low | There is evidence on the efficacy of nonmedical face masks is scarce and heterogeneous. | Weak in support | Use of nonmedical masks as recommended by the WHO or, in Spain, masks with UNE 0064-2:2020 or UNE 0065 specifications or any other tested in accredited laboratories and in adherence with current policy, is expected in individuals aged 6 years and older (and recommended from years) that are not infected by SARS-CoV-2. |
| Adverse effects of masks | |||
| Very low | The most frequent side effect is discomfort during use. There have been no reports of adverse events. | ||
| Risk of neonatal infection in infants born to SARS-CoV-2-positive mothers | |||
| Low | Vertical transmission of SARS-CoV-2 is very rare, and it has not been possible to establish the timing of transmission (intrauterine, intrapartum or postnatal). | ||
| Influence of the type of delivery in the vertical transmission of SARS-CoV-2 to newborns of positive mothers | |||
| Low | There is no difference in the SARS-CoV-2 infection status of newborns delivered vaginally versus those delivered by caesarean section. | Strong in support | Taking into account the costs and benefits, the lack of evidence on the advantages of caesarean delivery and the preference of families, it is recommended that the decision whether to have a caesarean or vaginal delivery be made based on obstetric criteria and not seeking to modify the risk of transmission. |
| Is it possible to breastfeed newborns of SARS-CoV-2-positive mothers? | |||
| Low | The scarce evidence available does not suggest that SARS-CoV-2 is transmitted through breast milk. | Strong in support | The promotion and maintenance of breastfeeding in newborns of mothers with SARS-CoV-2 infection is strongly recommended. |
| Use of preventive measures in the perinatal period to prevent transmission of SARS-CoV-2 to the newborn | |||
| Very low | The scarce evidence available does not allow estimation of the efficacy of preventive measures meant to reduce transmission of SARS-CoV-2 to newborns. | Weak in support | Taking into account the costs and benefits, the lack of evidence on the advantages and safety of not implementing preventive measures and the preference of families, in the case of SARS-CoV-2-positive mothers, contemplate maintaining the routine preventive measures that are currently recommended: rooming-in keeping a safe distance except when the infant is being breastfed, handwashing, and use of face masks while the mother remains infectious. |
| Schools and activities | |||
| Low | There is evidence of low quality that schools are not an important source of transmission and there is no certainty that their closure would decrease the incidence of disease in the general population or in the population under 18 years, or that it would have a significant impact on the percentage of severe cases (requiring ICU admission) or overall mortality due to COVID-19. | Weak against | Closure of schools and childcare centres as a preventive measure to decrease the incidence and severity of COVID-19 in the general population is not recommended. |
CI, confidence interval; RR, relative risk; WHO, World Health Organization.
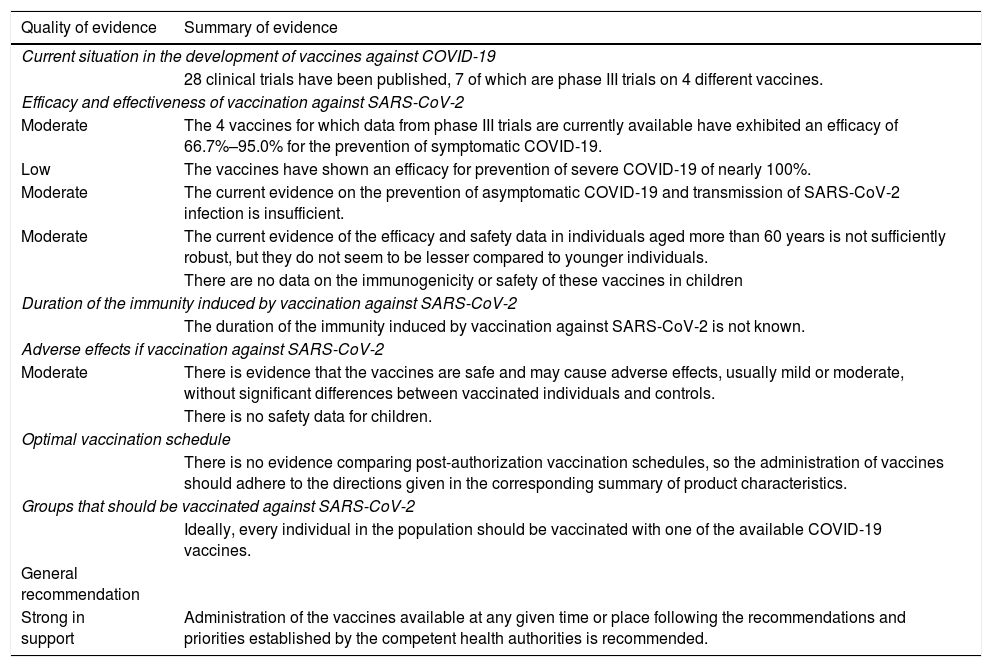
Quality of evidence and recommendations for vaccination against SARS-CoV-2.
| Quality of evidence | Summary of evidence |
|---|---|
| Current situation in the development of vaccines against COVID-19 | |
| 28 clinical trials have been published, 7 of which are phase III trials on 4 different vaccines. | |
| Efficacy and effectiveness of vaccination against SARS-CoV-2 | |
| Moderate | The 4 vaccines for which data from phase III trials are currently available have exhibited an efficacy of 66.7%–95.0% for the prevention of symptomatic COVID-19. |
| Low | The vaccines have shown an efficacy for prevention of severe COVID-19 of nearly 100%. |
| Moderate | The current evidence on the prevention of asymptomatic COVID-19 and transmission of SARS-CoV-2 infection is insufficient. |
| Moderate | The current evidence of the efficacy and safety data in individuals aged more than 60 years is not sufficiently robust, but they do not seem to be lesser compared to younger individuals. |
| There are no data on the immunogenicity or safety of these vaccines in children | |
| Duration of the immunity induced by vaccination against SARS-CoV-2 | |
| The duration of the immunity induced by vaccination against SARS-CoV-2 is not known. | |
| Adverse effects if vaccination against SARS-CoV-2 | |
| Moderate | There is evidence that the vaccines are safe and may cause adverse effects, usually mild or moderate, without significant differences between vaccinated individuals and controls. |
| There is no safety data for children. | |
| Optimal vaccination schedule | |
| There is no evidence comparing post-authorization vaccination schedules, so the administration of vaccines should adhere to the directions given in the corresponding summary of product characteristics. | |
| Groups that should be vaccinated against SARS-CoV-2 | |
| Ideally, every individual in the population should be vaccinated with one of the available COVID-19 vaccines. | |
| General recommendation | |
| Strong in support | Administration of the vaccines available at any given time or place following the recommendations and priorities established by the competent health authorities is recommended. |
Most of the published studies on the transmission of SARS-CoV-2 are observational and use different variables and assessment criteria, which did not allow a meta-analysis of the results. The available reviews are mostly narrative, and while there are a few systematic reviews (SRs), due to the characteristics of the studies that they included we did not believe it would be useful to analyse them in greater detail.
Although the infectious period seems to range from 2 days before the onset of symptoms to 8–10 days after (the pattern in asymptomatic individuals seems to be similar, but it is not well known), some studies have demonstrated that 44% of contagions occur while the primary case is asymptomatic.4 Different authors have found substantial variability in the transmission of SARS-CoV-2 depending on the context.5 The evidence shows that 10%–20% of primary cases are responsible for 80% of secondary cases. There are probably factors at play in transmission other than viral load. Transmission within the household is 10 times more frequent than outside the household.
International epidemiological data and 1 SR have found a risk of secondary transmission in the population aged under 22 years that is 44% lower compared to the adult population. Children are rarely the index case in their families or the cause of outbreaks.6
According to the World Health Organization (WHO), COVID-19 is often more severe in people who are older than 60 years or who have health conditions like high blood pressure, cardiovascular or lung problems, diabetes, obesity or cancer, although individuals of any age may develop severe COVID-19 or die from it.
We searched the literature for data on the risk of developing disease of any severity. The data were obtained from observational studies with a high risk of bias and a low or very low quality of evidence (Table 1).7 The only risk factor that seems supported by evidence of moderate quality is social inequity, which is measured through different indicators and that is significantly correlated to the incidence and mortality of COVID-19.8
In conclusion, although any unvaccinated individual is at risk of being infected, older individuals and individuals with underlying disease are at higher risk of severe COVID-19. The risk of transmission seems higher in adults, elderly individuals in residential facilities, household members of diseased individuals with a dry cough and groups of lower socioeconomic status. Weather and geographical factors may also play a role.
Clinical pictureGiven the heterogeneity of the selected studies, we made a qualitative synthesis of their results but not a meta-analysis. Table 2 presents the most relevant findings.
We included case series describing at least 10 microbiologically confirmed cases and data on symptoms, as well as SRs including studies of these characteristics. Due to the scarcity of neonatal data, we made an exception and included one SR that analysed isolated case reports and small case series.9 We excluded all the primary sources already included in the selected SRs and sources describing patients that were very likely to be included in larger case series. Most studies had a retrospective observational design and had been conducted in hospital settings (inpatient and emergency care) in the early months of the pandemic. These SRs also had methodological flaws and included heterogeneous results. There was a high risk of bias in all, tending to the underrepresentation of asymptomatic patients and overestimation of the severity of symptoms. Early in the pandemic, tests for microbiological confirmation were ordered nearly exclusively in patients with fever, respiratory symptoms or significant comorbidities, which may have resulted in overestimation of these features. We obtained likelihood ratios for the presence of specific symptoms and a positive reverse-transcriptase polymerase chain reaction (RT-PCR) test for SARS-CoV-2 from a study of greater quality conducted in the community in Canada that included 2463 cases diagnosed between April and September 2020. The best predictor was the combination of 3 symptoms: anosmia/ageusia, nausea/vomiting and headache.10 When it comes to the risk of hospitalization, the data are imprecise, probably due to the absence of uniform criteria for hospital admission and selection biases in different studies. The most consistent risk factors were age less than 1year, especially age less than 3 months, preterm birth and certain underlying diseases.11,12 The results of different studies regarding the risk of admission to the intensive care unit (ICU) were heterogeneous, and the most consistent factors were age less than 1 month and elevation of C-reactive protein.
We collected data on the multisystem inflammatory syndrome in children (MIS-C)13 from studies that included patients with this particular diagnosis, but the data from all other complications was obtained from general studies on the clinical features of SARS-CoV-2 infection. It is possible that instances of these variables were not documented in primary sources, even if they were actually present. Neurologic complications were analysed in a SR of data obtained from studies on the overall clinical presentation and course of the disease (not focused specifically on neurologic features).14
Diagnostic testsIn the early months of the COVID-19 pandemic, the ability to diagnose the disease accurately was of critical importance, so numerous studies analysed the different available RT-PCR techniques. In the months that followed, new diagnostic needs emerged, such as improving the turnaround time of tests, the availability of tests for screening and to conduct population seroprevalence studies or reducing the costs of testing, which resulted in the publication of many works focused on the validity of different types of samples and rapid PCR, antigen and antibody tests.
To establish the gold standard, we analysed data from studies on the correlation and the limits of detection of different RT-PCR tests,15 which were in vitro studies with an appropriate methodological design. When it came to rapid PCR and antigen tests, we searched for studies on their validity and accuracy, most of which, be it individual studies or studies included in meta-analyses, were limited on account of their design and methodology, especially in relation to the inclusion criteria and the reference standard.16 Furthermore, few studies included children and, when they did, their representation was all but negligible. We analysed one study on the validity of antigen tests focused exclusively on the paediatric population, with results that we considered of moderate-to-low quality due to low precision.
To evaluate the usefulness of quantitative SARS-CoV-2 antibody tests, we analysed data from 4 studies, one of which was a review included in a clinical practice guideline.17 All recommendations on the subject are based on evidence of very low quality, as most of the sources were case-control studies with a high risk of bias. The findings of the studies, including those included in meta-analyses, were heterogeneous, and there was little agreement in the definition of key concepts, such as the time elapsed from the onset of symptoms. On the other hand, since most of the studies analysed were conducted on the adult population, the evidence is also indirect.
Once the diagnosis has been established, the evidence in adults shows that certain clinical, laboratory or radiological characteristics may support the diagnosis or have prognostic value. The studies we included in the analysis to determine the indication of blood tests,18 chest radiograph, chest computed tomography19 or ultrasound20 in the paediatric population were observational studies with small samples and of very low quality. It is possible that new studies on the subject may improve the quality of the evidence, although we doubt that it will change the direction of the current recommendations (Table 3).
TreatmentSince the beginning of the pandemic caused by the novel coronavirus, the global scientific community has made an immense effort to determine which treatments are most appropriate. Still, the experimental evidence in the paediatric population is scarce. The searches we performed did not yield any paediatric randomised controlled trials (RCTs) for the treatments included in the analysis. For this reason, we selected RCTs in adults and paediatric studies irrespective of study design. The main treatment options considered in the analysis were hydroxychloroquine,21 steroid therapy,22 tocilizumab,23 hyperimmune plasma,24 immunoglobulin therapy and remdesivir.25
For each treatment, we performed a quantitative synthesis of critical outcome measures, mainly mortality, the most important outcome, and the need of mechanical ventilation. We also estimated other outcomes for some of the treatments, such as survival times or radiological progression.
We were unable to pool the data on the adverse effects of treatment due to differences in reporting between trials, although the safety profile of some of these drugs had already been established (hydroxychloroquine/steroids). Some of the evaluated treatments are not widely accessible (high cost and long duration of treatment), which results in an unfavourable cost-benefit ratio (tocilizumab).
Overall, the quality of the evidence was considered moderate for some treatments, due to inconsistencies, or in some cases low to very low, due to lack of precision and methodological limitations (remdesivir). We only found evidence to support the use of steroid therapy, and evidence against the use of all other treatments. We expect that as new studies are published, the estimated efficacy of some of these treatments will change and may require reconsidering the current recommendations (Table 4).
The evidence on COVID-19 in the paediatric population and specifically on paediatric patients with MIS-C26 came from observational studies, which outlined a series of therapeutic interventions the efficacy of which cannot be established at present. It seems reasonable to recommend that most paediatric patients with COVID-19 that are asymptomatic or have mild symptoms receive any necessary supportive care. Pharmacological treatment should be reserved for severe cases (chiefly manifesting with MIS-C). In the case of MIS-C, the evidence supports a weak recommendation of combined therapy with intravenous immunoglobulin and methylprednisolone.
PreventionMasksPhysical measures are currently the cornerstone of prevention of COVID-19 in the paediatric population. The evidence on the effectiveness of masks is scarce and usually refers to the use of surgical masks, FFP2 masks or equivalent. We did not find any studies devoted exclusively to the paediatric population, although children were included in some of the studies conducted in the community. The evidence on the effectiveness of masks for prevention of COVID-19 is equally poor, as most studies were conducted before the pandemic and focused on other respiratory viruses (influenza, SARS, MERS). Lastly, results regarding their effectiveness vary based on the study design: while clinical trials have not found evidence of effectiveness,27 observational studies have found an important protective effect.28 The low quality of the evidence does not allow drawing firm conclusions (Table 5). When it comes to safety, there have been no reports of significant adverse events.
Vertical transmission, breastfeeding and preventive measuresTo date, there is no evidence of SARS-CoV-2 vertical transmission to newborns. The conclusions are based on observational studies of moderate-to-low quality that differed in terms of the samples, methods and timing of testing. In nearly all studies, vertical transmission was an infrequent and uncertain event.29 We found no differences in transmission based on the type of delivery (vaginal/caesarean). There is no evidence that SARS-CoV-2 can be transmitted through breast milk, and there have been no differences in the reported positivity rate between breastfeed and formula-fed newborns.30 Where the newborn stays in hospital during the postpartum period, either rooming-in with the mother or separated from the mother, does not have an impact on the risk of neonatal infection.
School closuresStudies on the closure of schools published early in the pandemic based their predictions on the behaviour of other viruses, to which we must add the difficulty of isolating the effect of closing schools from the effects of all other nonpharmacological hygiene and social distancing measures that were implemented more or less simultaneously.31 Some of the most recent studies analyse outcomes based on the level of community transmission of the virus,32 and therefore recommendations could change during incidence peaks in the community. As is the case of any public health measure, when it comes to making recommendations, it is necessary to take into account all the costs and benefits to society of closing schools and childcare centres.
VaccinationThe publication of the findings of ongoing trials of the different vaccines that are being investigated has been spotty; for some vaccines, data have been published and kept the community abreast of developments in research, whereas for other vaccines no data have been published to date.33–36
In the European Union, 4 vaccines against COVID-19 had been authorised as of March 11, 2021: Comirnaty (Pfizer-BioNTech), the Moderna COVID-19 vaccine, the AstraZeneca COVID-19 vaccine and the Jannsen COVID-19 vaccine. At the time of this writing (April 23, 2021), there are data on the efficacy of 2 nonreplicating vaccines based on recombinant viral vectors and 2 vaccines based on messenger RNA, which is greater than 60% in all. This document does not include an assessment of the Janssen vaccine because data from clinical trials is not yet available.
The effectiveness data published to date are encouraging.37 In Spain, there is evidence of a decrease in the incidence of COVID-19 in nursing homes for the elderly, which were a priority group in the vaccination drive.
As occurs with other RNA viruses, there may be variants of SARS-CoV-2 that escape neutralization by antibodies, As of March 11, there are 3 variants of concern: B.1.1.7 (UK variant), B.1.351 (South Africa variant) and P.1 (Brazil variant).38
The overall quality of the evidence on vaccines against SARS-CoV-2 is moderate, but this is too low to support vaccination of children, as the evidence is indirect, based on RCTs performed in adults.
The evidence is from well-designed, blinded RCTs, but these trials have not yet completed the follow-up. Some groups are underrepresented in RCTs, like individuals aged more than 55 years or with diseases, and other groups, such as children and pregnant women, have been completely excluded.
Vaccines against COVID-19 are being authorised on an urgent basis due to the severity of the pandemic. In general, the population seems willing to undergo vaccination, although there is unprecedented influence from information delivered through the mass media and social networks, with messages that are at times confusing, contradictory or simply false.
Although the data on the effectiveness of the vaccines are still preliminary, given the efficacy observed in trials and the generally mild-to-moderate severity of its adverse effects, the benefits seem to outweigh the risks and support vaccination of the population (Table 6).
Due to the scarcity of doses and logistic difficulties, the health care authorities have established priority groups to immunise different segments of the population in a stepwise approach.
Pilar Aizpurua Galdeano. Centro de Salud Ondarreta. San Sebastián.
María Aparicio Rodrigo. Centro de Salud Entrevías. Madrid.
Jaime Javier Cuervo Valdés. Centro de Salud Ciudad Jardín. Badajoz.
Ana Isabel Díaz Cirujano. Centro de Salud Rosa Luxemburgo. Madrid.
María Jesús Esparza Olcina. Madrid.
María Mercedes Fernández Rodríguez. Centro de Salud Potes. Madrid.
Paz González Rodríguez. Centro de Salud Barrio del Pilar. Madrid
Blanca Juanes de Toledo. Centro de Salud Collado Villalba. Madrid.
Victoria Martínez Rubio. Centro de Salud Los Fresnos. Torrejón de Ardoz, Madrid.
Eduardo Ortega Páez. UGC Macarena. Distrito Metropolitano. Granada.
Leo Perdikidis Olivieri. Centro de Salud Juncal. Torrejón de Ardoz, Madrid.
Enrique Rodríguez-Salinas Pérez. Centro de Salud Colmenar Viejo Norte. Madrid.
Juan Ruíz-Canela Cáceres. Seville.
María Salomé Albi Rodríguez. Hospital Universitario 12 de Octubre. Madrid.
Sergio Flores Villar. Hospital Universitario Mutua de Terrassa. Barcelona.
Álvaro Gimeno Díaz de Atauri. Hospital Universitario 12 de Octubre. Madrid.
Javier González de Dios. Hospital General Universitario de Alicante. Alicante.
Rafael Martin Massot. Hospital Regional Universitario de Málaga. Malaga.
Carlos Ochoa Sangrador. Complejo Asistencial de Zamora. Zamora.
Giordano Pérez Gaxiola. Hospital Pediátrico de Sinaloa Dr. Rigoberto Aguilar Pico. México.
Begoña Pérez-Moneo Agapito. Hospital Universitario Infanta Leonor. Madrid.
María José Rivero Martín. Hospital Universitario de Fuenlabrada, Madrid.
Please cite this article as: González Rodríguez P, Pérez-Moneo Agapito B, Albi Rodríguez MS, Aizpurua Galdeano P, Aparicio Rodrigo M, Fernández Rodríguez MM, et al. COVID-19 en pediatría: valoración crítica de la evidencia. An Pediatr (Barc). 2021;95:207.
Appendix A lists the members of the Working Group on Evidence-Based Paediatrics of the AEP and AEPap that participated in the development of this document.