Haemolytic disease of the foetus and newborn (HDFN) affects 3–80 per 100 000 patients each year. Although the most frequent cause of HDFN is Rhesus D (RhD) antigen alloimmunization, other red blood cell antigens can cause the disease, for instance, through anti-c isoimmunization. When the incompatibility is severe, it can cause foetal anaemia, hydrops fetalis and even intrauterine death. In neonates, phototherapy is the most widely used treatment in mild and moderate cases. In severe cases, intravenous immunoglobulin (IVIG) and exchange transfusion may be useful to prevent bilirubin encephalopathy.1
We present the case of a preterm newborn with HDFN caused by an uncommon type of antibodies (anti-c). The patient experienced an insidious course that required treatment with IVIG and exchange transfusion. She then developed necrotising enterocolitis (NEC).
The patient was a newborn girl admitted to our hospital due to jaundice with onset within 24h of birth. She was the second child of a healthy mother aged 37 years that had received a diagnosis at 24 weeks of gestation of Rh isoimmunization due to anti-c antibodies. Foetal ultrasound examinations were carried out, which did not detect abnormalities in the foetus, with the exception of mild anaemia at 30 weeks of gestation.
The patient was born at 35+1 weeks of gestation. Her blood type was A Rh+ and the direct Coombs tests was positive, evincing the presence of anti-c antibodies. At 24h post birth, the physical examination of the patient was normal with the exception of jaundice. Laboratory tests confirmed hyperbilirubinaemia (total bilirubin, 13.5mg/dL; normal range, <8mg/dL) in the absence of anaemia (haemoglobin, 16.7g/dL), leading to initiation of intensive phototherapy for 14 days with a positive outcome. Bilirubin levels remained below the exchange range. Other tests were performed to rule out alternative diagnoses to hyperbilirubinaemia.
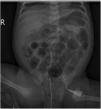
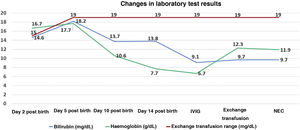
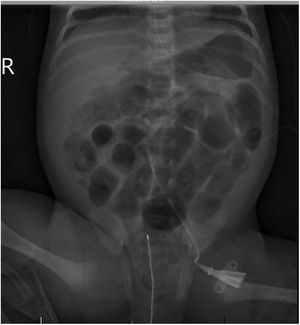
At 14 days post birth, the patient developed anaemia (haemoglobin, 7.7g/dL; normal range, 12.5–20.5), leading to administration of IVIG (1g/kg IV over 6h). The patient remained haemodynamically stable before and during treatment. The anaemia progressed despite IVIG (haemoglobin, 6.7g/dL 12h after immunoglobulin administration), so the patient underwent partial exchange transfusion (80cc/kg) delivered through a central venous catheter (left femoral vein). There were no adverse events associated with the transfusion. The haemoglobin concentration increased to 12.3g/dL (Fig. 1). A few hours after the transfusion was completed, the patient exhibited general deterioration with abdominal distension and bilious vomiting. She required endotracheal intubation and haemodynamic support with dopamine. The findings of the abdominal radiograph were suggestive of NEC (Fig. 2). The patient underwent an urgent laparotomy that confirmed the diagnosis. This was followed by performance of an end ileostomy with removal of the distal ileum and ascending colon. After the surgery, the patient recovered gradually and was discharged at day 30.
Minor blood group incompatibility has been found to be responsible for 3%–5% of cases of haemolytic jaundice in neonates.2 Antigen c is one of the most immunogenic antigens, following antigen D, with immunization usually developing antenatally and associated with a risk of moderate to severe HDFN.3
Haemolytic disease of the foetus and newborn can cause sustained anaemia, possibly with antenatal onset, causing tissue hypoperfusion and particularly affecting the gastrointestinal tract.4 In addition, it is associated with an increased risk of preterm birth, and enterocolitis is more frequent in preterm newborns.
Administration of immunoglobulin is recommended for treatment of hyperbilirubinaemia refractory to phototherapy and also of anaemia secondary to HDFN. Intravenous immunoglobulin also seems to be associated with the development of NEC, as it causes an increase in blood viscosity that may give rise to the formation of microthrombi and a reduced mesenteric blood flow. Figueras-Aloy et al. described the association between the administration of immunoglobulin with haemolytic disease and the development of NEC.5
The association between the administration of blood products and NEC is also well established, with NEC usually appearing between 24 and 48h post transfusion.6 The factors that promote enterocolitis are inflammation secondary to the infusion of red blood cells, the decreased capacity of haemoglobin in banked red blood cells to delivery oxygen compared to foetal haemoglobin and the increased viscosity of the blood.
Haemolytic disease of the foetus and newborn, IVIG therapy and exchange transfusion are independently associated with the development of NEC. In our patient, anaemia became severe from 14 days post birth, although it is not possible to determine whether earlier administration of IVIG or exchange transfusion could have prevented NEC. At any rate, based on the sequence of events, IVIG administration seems the most plausible explanation for NEC, as necrosis was already present at the time of surgery, indicating that ischaemia had started at least 12h before the operation.
In conclusion, neonates with HDFN are at increased risk of requiring IVIG and exchange transfusion, both associated with the development of NEC, and therefore require careful monitoring.
FundingThis research did not receive any external funding.
Previous presentation: This study was presented at the XXVIII Congress of the Sociedad Española de Neonatología in October 2021with the title “Enterocolitis necrotizante tras la administración de inmunoglobulina intravenosa y transfusión de hematíes en pretérmino tardío con enfermedad hemolítica anti-c”.