Premature pubarche (PP) is generally thought to be a benign condition, but it can also be the first sign of underlying disease.
ObjectiveTo analyse the aetiology and the evolution of the anthropometric, analytical and metabolic risk parameters of a group of patients with PP.
Material and methodsA descriptive and analytical retrospective study of 92 patients affected by PP. Anthropometry, analyses, bone age and indicators of lipid metabolism were all evaluated.
ResultsThe sample included 92 patients with PP (67 female and 25 male), with a mean age of 7.1±0.6 for girls and 8.3±0.7 for boys. Small for gestational age was recorded in 7.7%. There was an accelerated bone age (1.20±0.1 years). A total of 21 patients were classified as idiopathic (23%), 60 as idiopathic premature adrenarche (65%), and 11 with non-classic congenital adrenal hyperplasia (12%). Puberty was reached early (11+0.9 years old in boys and 9.9±0.8 in girls), as was menstruation age (11.8+1.1 years old), P<.001. The stature finally reached was close to their genetic stature. There is a positive correlation between body mass index, blood glucose and LDL cholesterol, as well as a tendency towards hyperinsulinaemia.
ConclusionsThe present study shows that PP is a benign condition in the majority of cases, but non-classic congenital adrenal hyperplasia (12%) is not uncommon. Menstruation and puberty started early and bone age was accelerated. Growth was normal, and more or less in line with genetic size. PP associated with obesity is linked with analytical variations of metabolic risks.
La pubarquia precoz (PP) es generalmente considerada como una enfermedad benigna, pero puede ser el primer signo de una enfermedad subyacente.
ObjetivoAnalizar la etiología y la evolución de parámetros antropométricos, analíticos y de riesgo metabólico, en pacientes con PP.
Material y métodosEstudio retrospectivo, descriptivo y analítico, de 92 pacientes afectos de PP. Se evaluaron medidas antropométricas y analíticas, la edad ósea y marcadores de metabolismo lipídico.
ResultadosMuestra de 92 pacientes (67 mujeres y 25 varones) con PP, con una edad media de 7,1±0,6 años las mujeres y 8,3±0,7 los varones. El 7,7% fueron pequeños para la edad gestacional. La edad ósea estaba adelantada (1,2±0,1 años). Veintiún pacientes fueron clasificados como PP idiopática (23%), 60 como adrenarquia precoz idiopática (65%) y 11 como hiperplasia suprarrenal congénita no clásica (12%). La pubertad se mostró adelantada respecto a la media (11±0,9 años en varones versus 9,9±0,8 años en mujeres), así como la edad de la menarquia (11,8±1,1 años), p<0,001. La talla final alcanzada es próxima a la talla genética. Existe una correlación positiva entre el Z-score del índice de masa corporal, la glucemia y el colesterol LDL, así como una tendencia a la hiperinsulinemia.
ConclusionesEl presente estudio demuestra como la PP en la mayoría de los casos supone una patología benigna, no siendo infrecuente la hiperplasia suprarrenal congénita no clásica (12%). Estos pacientes presentaron un adelanto puberal, de la edad ósea y de la menarquia. El crecimiento fue adecuado, alcanzando prácticamente su talla genética. La PP asociada a obesidad presenta alteraciones analíticas de riesgo metabólico.
The term pubarche refers to the development of pubic hair, which may be an isolated event or be accompanied by axillary hair, oily skin, acne or adult-like body odour. This process is considered premature when it occurs before age 8 years in girls and 9 years in boys.1–4 Each of these clinical features may appear in isolation in the context of physiological adrenarche, which starts at around age 6–8 years.5 Premature adrenarche (PA) refers to the premature production of adrenal androgens and is the most frequent cause of pubarche. Its prevalence varies depending on the criteria applied for its definition and on ethnicity, and its incidence is higher in African American children.6 There is a clear female predominance (female to male ratio, 9 or 10:1),7 and is characterised by occurring independently of puberty. The best marker of adrenarche is dehydroepiandrosterone sulphate (DHEA-S): levels of this metabolite greater than 40–50μg/dL are considered indicative of its onset.1,2,5
Premature pubarche (PP), in which pubarche is not accompanied by any other sign of puberty, marked virilization or an abnormally advanced bone maturation (≥2 years), is considered a normal variation.5,8 However, there is debate as to whether it should be considered a separate clinical entity and included in the category of prepubertal hyperandrogenism, as affected girls may exhibit prepubertal hyperinsulinism and are at increased risk of ovulatory dysfunction, functional ovarian hyperandrogenism, dyslipidaemia and obesity in adolescence.4,5
Premature adrenarche and premature pubarche are not equivalent terms, even though they are sometimes used interchangeably. No unanimous criteria have been established in the literature to defined variations of normal such as idiopathic premature adrenarche (IPA) or idiopathic premature pubarche (IPP). Some authors consider that IPA should be defined exclusively by clinical criteria (development of pubic and/or axillary hair and/or increase of body odour of apocrine origin) before age 8 years in girls and 9 years in boys, after ruling out other diseases that can cause hyperandrogenism (tumours, adrenal hyperplasia).4,7 Others define IPA based on the presence of clinical criteria combined with biochemical criteria (DHEA-S levels greater than those found in prepubertal children, with a cut-off value of 40μg/dL), and IPP as pubarche occurring before age 8 years in girls and 9 years in boys after ruling out all other possible causes of hyperandrogenism, with DHEA-S values of less than 40–50μg/dL.1,2
The exact mechanisms underlying this phenomenon are unknown, but it seems that the adipose tissue2 and a hypersensitivity of the hair follicle to steroid hormones4,9,10 may play a significant role in its presumably multifactorial aetiology.
We conducted a study on a group of patients with PP, analysing their clinical manifestations through adulthood and exploring the aetiology of PP and its association with anthropometric and laboratory values and metabolic risk factors.
Patients and methodsWe conducted a retrospective, observational, descriptive and inferential study in patients with PP managed at the paediatric endocrinology unit of a tertiary care hospital between years 2000 and 2015.
We included a total of 92 patients that met the following criteria:
- •
Inclusion criteria:
- -
Isolated pubarche in girls aged <8 years or boys aged <9 years.
- -
Follow-up in the clinic until linear growth was complete.
- -
Classification based on physical examination, hormone levels and bone age:
- a)
IPP (normal DHEA-S levels).
- b)
IPA (DHEA-S levels >50μg/dL).5
- c)
Nonclassic congenital adrenal hyperplasia (NCAH; ACTH 17-hydroxyprogesterone [17-OHP] level after adrenocorticotropic hormone [ACTH] stimulation test >10ng/mL).
- •
Exclusion criteria:
- -
Absence of any of the inclusion criteria.
- -
Signs of puberty: thelarche in girls and testicular volume ≥4mL in boys.
- -
Hyperandrogenism due to a tumour, brain disorder or multiple malformation syndrome that may affect growth or development.
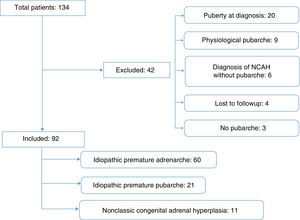
The initial sample consisted of 134 patients referred for clinical suspicion of PP. We excluded 44 patients, 38 because they did not meet the inclusion criteria and 4 because we were unable to access their medical records (Fig. 1).
We reviewed the health records of the patients to collect data on their perinatal history, auxologic measurements, laboratory values and radiologic findings during their follow-up. The study was approved by the clinical research ethics committee of our hospital.
We collected data on the weight and length at birth and defined small for gestational age (SGA) as birth weight and/or length at least 2 standard deviations (z-score <−2) below the mean of the reference population.11
We analysed anthropometric variables (weight, height, body mass index [BMI] and pubertal stage). We calculated the corresponding z-scores using Spanish reference tables.12 Pubarche was classified according to the 5 Tanner stages,13 including every patient with at least stage II development. We defined onset of puberty based on Tanner stage II13 as thelarche in girls and a testicular volume of at least 4mL, measured with a Prader orchidometer, in boys.
We also analysed the age at menarche and the time elapsed from the diagnosis of pubarche to the onset of puberty and the time elapsed from the diagnosis of pubarche and the onset of puberty to menarche.
For all patients, we calculated the chronological age (CA) and the bone age (BA) (applying the approach of Greulich and Pyle14) and predicted the final adult height (using the method of Bayley and Pinneau15). We defined final height as the height achieved with a BA greater than 15 years in boys and greater than 14 years in girls and/or a growth rate of less than 2cm/year.12
Fasting blood specimens were collected in the morning to measure serum levels of 17-OHP (ng/mL) and DHEA-S (μg/dL). Values of DHEA-S greater than 50μg/dL are indicative of PA. Patients with 17-OHP levels greater than 2ng/mL were further assessed with the ACTH stimulation test (with intravenous administration of 250μg/m2 of synthetic ACTH and extraction of blood samples 30 and 60minutes after to measure levels of 17-OHP and cortisol); values of 17-OHP greater than 10ng/mL at either time point after stimulation are indicative of NCAH, which needs to be confirmed by genetic testing. Blood tests were performed during the follow-up to determine serum levels of glucose (mg/dL), insulin (μIU/mL), total cholesterol (mg/dL), low-density lipoprotein cholesterol (LDL-C, mg/dL) and high-density lipoprotein cholesterol (HDL-C, mg/dL). We calculated the Homeostasis Model Assessment insulin resistance (HOMA-IR) index as an indirect marker of insulin resistance.16
We performed a descriptive and inferential statistical analysis using the software SPSS 23.0 for Windows. We have expressed descriptive results as measures of central tendency (mean) and of dispersion (standard deviation [SD]). We used parametric tests to compare normally-distributed quantitative variables (Student t test for 2 variables, and ANOVA for more than 2 variables), and nonparametric tests to compare quantitative variables that did not meet the assumption of normality (Mann–Whitney U test for 2 independent continuous variables, and Kruskal–Wallis for more than 2 independent continuous variables). We analysed repeated measurements with the Student t test for paired samples and used the Pearson correlation coefficient to assess the linear correlation between quantitative variables. We defined statistical significance for all the tests as a P-value of less than .05.
ResultsWe analysed data for 92 patients with PP, 72.8% female and 27.2% male.
The mean age at pubarche was 8.3±0.7 years in the boys and 7.1±0.6 in the girls. The mean age at onset of puberty was 11±0.9 years (range, 9.7–12.5 years) in boys and 9.9±0.8 years in girls (range, 8.2–11.9 years); the mean age at menarche was 11.8±1.1 years. The time elapsed between onset of puberty and menarche was 2.2±1.2 years.
We found a slightly advanced BA (BA/CA ratio >1) in all patients and in each of the periods during the follow-up, with a range of 1.1±0.1 to 1.4±0.9.
We did not find any differences in the weight, height or BMI z-scores at any of the assessment time points compared to the reference population.
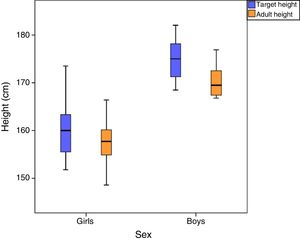
The mean final height was 170.4±3.7cm in boys (z-score, 1.5±0.9) and 157.6±4.6cm in girls (z-score, −0.2±1); the difference between the target height and the final height was greater in boys (4.5±4.1cm compared to 2.3±3.6cm in girls) (Fig. 2).
When it came to diagnosis, we classified 60 patients (65%) as having IPA, 21 (23%) as having IPP and 11 (12%) as having NCAH. Patients with NCAH had a lower mean age at pubarche compared to patients with IPA (5.7±1.9 years vs 7.9±1.1 years, P=.01), and a more advanced BA (1.7±0.9 years, P=.005). We did not find differences in the age at onset of puberty between the different groups, or in the mean age at menarche (IPP group, 11.5±0.5 years; IPA group, 11.4±0.9 years; NCAH group, 11.9±0.6 years). The mean time elapsed from pubarche to menarche was shorter in patients with IPA (4.4±1.9 years) compared to patients with IPP (6.1±0.9 years) (P=.045), with a smaller difference between groups in the time elapsed between the onset of puberty and menarche (2±1.1 years in the IPA group vs 2.7±1.9 years in the IPP group; P>.05) (Table 1).
Auxologic values at different stages by final diagnosis.
| Idiopathic premature pubarche | Idiopathic premature adrenarche | Nonclassic congenital adrenal hyperplasia | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean±SD | n | Mean±SD | n | Mean±SD | |||
| Onset of pubarche | CA | (years) | 21 | 7.1±2.3 | 60 | 7.9±1.1 | 11 | 5.7±1.9** |
| Weight | (kg) | 21 | 32.1±9.3 | 60 | 33.6±8 | 11 | 27.5±5.8 | |
| z-score | 21 | 0.6±0.7 | 60 | 0.5±0.9 | 11 | 0.7±1 | ||
| Height | (cm) | 21 | 130.3±18.4 | 60 | 133.1±8.6* | 11 | 123.4±10.4* | |
| z-score | 21 | 0.6±0.7 | 60 | 0.5±0.9 | 11 | 0.7±1 | ||
| BMI | (kg/m2) | 21 | 18.6±2.7 | 60 | 18.8±2.6 | 11 | 17.9±2.3 | |
| z-score | 21 | 0.5±1 | 60 | 0.4±0.8 | 11 | 0.6±1 | ||
| BA | (years) | 21 | 9.2±1.8 | 60 | 9.7±1.6 | 11 | 8.4±2.1 | |
| BA/CA | (years) | 21 | 1.1±0.1 | 60 | 1.1±0.1 | 11 | 1.7±0.9** | |
| PG | (cm) | 21 | 167±11.7 | 60 | 165.1±10.8 | 11 | 163.2±8.7 | |
| z-score | 21 | 0±1.2 | 60 | −0.5±1.2 | 11 | −0.6±1.1 | ||
| Onset of puberty | CA | (years) | 21 | 10.2±0.8 | 60 | 10.4±0.9 | 11 | 10±0.9 |
| Weight | (kg) | 21 | 40±6.5 | 60 | 41±8.7 | 11 | 40.3±10.9 | |
| z-score | 21 | 0.3±0.6 | 60 | 0.4±0.8 | 11 | 0.5±0.9 | ||
| Height | (cm) | 21 | 144.2±8.5 | 60 | 143.1±7.8 | 11 | 140.5±9.5 | |
| z-score | 21 | 0.5±0.9 | 60 | 0.2±1 | 11 | 0.1±0.9 | ||
| BMI | (kg/m2) | 21 | 19.2±2.4 | 60 | 19.9±2.8 | 11 | 20.2±3.3 | |
| z-score | 21 | 0.2±0.7 | 60 | 0.4±0.8 | 11 | 0.6±1 | ||
| BA | (years) | 21 | 10.9±1* | 60 | 11.7±0.9* | 11 | 11.4±1.2 | |
| BA/CA | (years) | 21 | 1±0.1* | 60 | 1.2±0.1* | 11 | 1.2±0.1 | |
| PG | (cm) | 21 | 164.6±11.4 | 60 | 162±9 | 11 | 160.4±11.1 | |
| z-score | 21 | −0.2±1.3 | 60 | −0.9±1 | 11 | −1.2±1 | ||
| Intervals | Pubarche-puberty | (years) | 21 | 3.4±1.9 | 60 | 2.5±1.2 | 11 | 3.9±3 |
| Pubarche-menarche | (years) | 17 | 6.1±0.9 | 42 | 4.4±0.9* | 8 | 5.8±1.2 | |
| Puberty-menarche | (years) | 17 | 2.7±1.9 | 42 | 2±1.1 | 8 | 1.9±0.5 | |
| Last checkup | CA | (years) | 10 | 13.2±1.7 | 20 | 13.4±1 | 5 | 16.6±2*** |
| Weight | (kg) | 10 | 51±4 | 20 | 55.5±9.5 | 5 | 57.1±10.7 | |
| z-score | 10 | 0.2±0.4 | 20 | 0.5±1 | 5 | 0±0.6 | ||
| Height | (cm) | 10 | 156.5±8 | 20 | 158.8±7 | 5 | 164.3±8.1 | |
| z-score | 10 | 0.0±1.3 | 20 | 0.1±1.2 | 5 | −0.1±1.1 | ||
| BMI | (kg/m2) | 10 | 21±2.7 | 20 | 21.9±3.2 | 5 | 21±2.2 | |
| z-score | 10 | 0.2±0.7 | 20 | 0.4±0.9 | 5 | 0.3±0.8 | ||
| BA | (years) | 8 | 14±0.8 | 20 | 14.5±1 | 1 | 15 | |
| BA/CA | (years) | 8 | 1.1±0.1 | 20 | 1.1±0.1 | 1 | 1.1 | |
| PFH | (cm) | 8 | 161.6±7.6 | 20 | 164.3±9.1 | 1 | 175.4 | |
| z-score | 8 | −0.6±0.9 | 20 | −0.4±0.9 | 1 | −0.1 | ||
| TH-FH | (cm) | 10 | 2.2±5.2 | 20 | 5±5.7 | 5 | 3.9±4 | |
| z-score | 10 | −1.1±1.0 | 20 | −0.8±0.9 | 5 | −0.1±0.8 | ||
BA, bone age; BA/CA, bone age/chronological age ratio; BMI, body mass index; CA, chronological age; FH, final height; PFH, predicted final height; SD, standard deviation; TH, target height.
Of the 11 children with NCAH, 9 (82%) had homozygous mutations (Val281Leu/Val281Leu) and 2 had heterozygous mutations (Val281Leu and Pro453Ser). All of them required treatment with hydrocortisone during the follow-up due to accelerated bone maturation.
The mean serum levels of glucose, total cholesterol, LDL-C and HDL-C were within normal ranges. We found a mild elevation of insulin levels in all 3 groups (IPP group, 10.9±6.3μIU/mL; IPA group, 10±7.9μIU/mL; NCAH group, 10.7±5.5μIU/mL) (Table 2). We did not find high HOMA-IR values in any of the diagnostic groups (range, 2.1±0.1–2.3±0.2).
Laboratory values by final diagnosis.
| IPP (n=21) | IPA (n=60) | NCAH (n=11) | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean±SD | n | Mean±SD | n | Mean±SD | |||
| Blood glucose | mg/dL | 8 | 86.8±10.6 | 44 | 80±7.4 | 11 | 79.7±9.4 | |
| Insulin | μIU/mL | 6 | 10.9±6.3 | 21 | 10±7.9 | 6 | 10.7±5.5 | |
| HOMA | 6 | 2.3±0.2 | 21 | 2±0.1 | 6 | 2.1±0.1 | ||
| Total cholesterol | mg/dL | 8 | 167.1±41.8 | 42 | 164.1±22.5 | 11 | 176.7±31 | |
| LDL-C | mg/dL | 7 | 106±36.8 | 24 | 93.9±19.5 | 10 | 99.3±25.7 | |
| HDL-C | mg/dL | 7 | 47.3±10.7 | 24 | 53.9±7.9 | 10 | 54.8±10.9 | |
| 17-OHP | ng/mL | 20 | 0.6±0.4 | 59 | 0.8±0.8 | 11 | 14.6±14.9 | |
| DHEA-S | μg/mL | 21 | 0.4±0.1 | 60 | 1.2±0.7 | 11 | 1.7±1.4 | |
| ACTH test | Baseline 17-OHP | ng/mL | 0 | 16 | 1.3±0.9 | 10 | 10.6±5.1 | |
| Peak 17-OHP | ng/mL | 0 | 16 | 4.5±3.2 | 10 | 28.8±23 | ||
DHEA-S, dehydroepiandrosterone sulphate; HDL-C, high-density lipoprotein cholesterol; HOMA, Homeostasis Model Assessment index; IPA, idiopathic premature adrenarche; IPP, idiopathic premature pubarche; LDL-C, low-density lipoprotein cholesterol; NCAH, nonclassic congenital adrenal hyperplasia; SD, standard deviation; 17-OHP, 17-hydroxyprogesterone.
In the sample under study (N=92), 6.2% of patients (n=6) had a BMI z-score of 2 or greater at the time pubarche was diagnosed. We found a positive correlation of the serum glucose and LDL-C levels with the BMI z-score (Table 3).
Metabolic risk factors and their correlation with the body mass index.
| Variable dependent | Variable independent | R | P |
|---|---|---|---|
| Blood glucose (mg/dL) | BMI (kg/m2) | 0.296 | NS |
| z-score BMI | 0.252 | .046 | |
| Insulin (μIU/mL) | BMI (kg/m2) | −0.224 | NS |
| z-score BMI | −0.239 | NS | |
| Total cholesterol total (mg/dL) | BMI (kg/m2) | 0.105 | NS |
| z-score BMI | 0.174 | NS | |
| LDL-C (mg/dL) | BMI (kg/m2) | 0.208 | NS |
| z-score BMI | 0.318 | .043 | |
| HDL-C (mg/dL) | BMI (kg/m2) | 0.095 | NS |
| z-score BMI | −0.032 | NS |
BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NS, not statistically significant; P, P-value; R, correlation coefficient.
Statistically significant results with P<.05 are presented in italics.
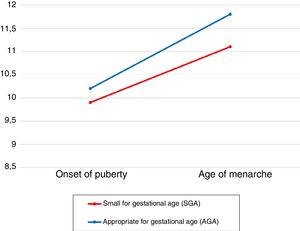
Of all patients, 7.7% (n=7) had been born SGA (birth weight, 2.1±0.6kg; birth length, 46.9±2.3cm). The mean age at onset of puberty (9.9±0.6 years) and the mean age of menarche (11.1±1.5 years) were lower in patients born SGA compared to patients whose birth size was appropriate for gestational age (AGA) (10.2±1 years vs 11.8±1.2 years; P>.05) (Fig. 3). The mean time elapsed from pubarche to menarche (3.8±2 years in the SGA group vs 5.2±2.2 years in the AGA group) and the mean time from onset of puberty to menarche (1.4±0.4 in the SGA group vs 2.2±1.2 years in the AGA group) were shorter in the SGA group (P>.05). We did not find differences in final height (159.8±7.6cm in the SGA group vs 161.1±5cm in the AGA group). Serum levels of DHEA-S (1.2±0.4μg/mL), 17-OHP (0.7±0.3ng/mL) and insulin (10.1±2.6μIU/mL) were similar in the SGA group and the AGA group (1.1±0.9μg/dL, 2.6±7.1ng/mL and 10.6±6μIU/mL, respectively). The mean body mass index z-score was also similar in both groups (SGA, 0.1±0.6; AGA, 0.5±0.9; P>.05).
DiscussionPremature pubarche is a frequent reason for consultation in paediatric endocrinology and exhibits a marked female predominance (72.8% of girls vs 27.2% of boys). The female-to-male ratio of patients affected by PP is of 9 or 10 to 1, but there are studies like the one conducted by Balducci et al.17 where the ratio is 4:1, similar to the ratio found in our study, which can probably be explained by our sample consisting of patients referred to a specialty clinic for assessment of suspected abnormalities of growth or development.
Idiopathic premature adrenarche is the most frequent cause of PP (65%).1,3 The mean age at diagnosis was 8.3±1.6 years in boys and 7.1±1.6 years in girls. The BA was slightly advanced in all patients (BA/CA ratio >1; range, 1.1±0.1 to 1.4±0.9), which was consistent with the reports of previous studies.5,18,19
When it came to the longitudinal changes in auxologic variables, and compared to the reference population,12 we found an early onset of puberty both in boys (11±0.9 vs 12.3±1.1 years) and girls (9.9±0.8 vs 10.7±1 years), and also a younger mean age of menarche (11.8±1.1 vs 12.6±1 years). In spite of this, the duration of puberty in our sample (time elapsed between the onset of puberty and menarche) was similar to the one observed in the reference population (2.2±1.2 vs 2.1±1 years). These data were consistent with the findings of the study of Ibáñez et al.20 of a sample of European girls with PA, with a mean age at onset of puberty of 9.7±0.9 years and a mean age at menarche of 12.0±1 years.
Both boys and girls achieved final heights close to their target heights, with minimal differences between the two (4.5±4.1cm in boys and 2.3±3.6cm in girls). This was consistent with the data of previously published reviews, which conclude that the acceleration of bone maturation caused by PA does not lead to significant decreases in final height, and that final heights are strongly correlated to the linear growth predicted at the time of diagnosis and at onset of puberty.2,5,20,21
On the other hand, it is important to measure hormone levels in addition to the assessment of clinical variables to establish the diagnosis. In our sample, 12% of patients had NCAH, which was within the observed range of the proportion of cases of PP where PP may be the initial manifestation of NCAH.22,23
Children in the three diagnostic groups (IPP, IPA e NCAH) exhibited a moderately advanced BA (BA/CA ratio, 1–2). Although the mean time elapsed from pubarche to menarche was significantly shorter in patients with IPA (4.4±1.9 years compared to 6.1±0.9 years in patients with IPP), the difference in the mean time elapsed from onset of puberty to menarche was not as big (IPA, 2±1.1 years; IPP, 2.7±1.9 years). This was consistent with the data for the reference population12 (mean time from onset of puberty to menarche, 2.1±1 years), which leads us to conclude that the time elapsed between onset of puberty and menarche in patients with PP is the same as the time elapsed in the general population, independently of the final diagnosis.
The mean serum levels of glucose, total cholesterol, LDL-C and HDL-C were within normal ranges in prepubertal patients,24 but insulin levels were elevated. The data reported in the literature in regard to dyslipidaemia and hyperinsulinemia is contradictory. The study by Ibáñez et al.25 reported elevation of both insulin and markers of lipid metabolism, and a higher prevalence of metabolic syndrome in girls with PP compared to the control population. Other studies26 have only found a mild elevation of insulin levels and lipid levels similar to those of controls in patients with PA, and concluded that insulin resistance is a risk factor for metabolic syndrome independent of obesity in patients with PA. In our study, we found that abnormal levels associated with metabolic risk, such as high blood glucose and LDL-C levels, were found in patients with PP that were overweight or obese.
When it came to the subset of patients born SGA (7.7%), and contrary to the findings of other studies,27,28 we did not find higher values of insulin or the BMI z-score at the time of diagnosis compared to the AGA group. In agreement with the results of the study by Boonstra et al.,29 DHEA-S levels were similar to those of the rest of the patients. As occurred in the study conducted by Ibáñez et al.,25 we found that patients born SGA that developed PP tended to have an earlier onset of puberty (9.9±0.6 years), a lower mean age of menarche (11.1±1.5 years), with a shorter time elapsed from onset of puberty to menarche (1.4±0.4 years), and a slightly shorter final height compared to patients born AGA.
In conclusion, PP is a benign condition in most cases, as demonstrated by our study. Patients with PP tend to exhibit well-defined clinical characteristics, such as an earlier onset of puberty (more marked in males) and menarche, an advanced BA, adequate growth that will allow them to achieve a final height close to their target heights, and laboratory abnormalities associated with increased metabolic risk (high blood glucose and LDL-C levels) and overweight/obesity. Based on our findings, it is not rare for NCAH to be diagnosed following the presentation of PP (12%), and these patients are the patients with PP that have the most marked features, such as an even younger age at pubarche and a more advanced BA at diagnosis, and therefore those in whom NCAH should be suspected. The final diagnosis of NCAH requires measurement of adrenal hormone levels and confirmation by genetic testing. For the above reasons, and even though PP does not require specific endocrinological treatment, follow-up of these patients is recommended due to the risk of early onset of puberty and future metabolic syndrome.
LimitationsIt is possible that the data of our study are affected by selection bias, as the sample consisted solely of patients with PP deemed to require follow-up at a specialised paediatric endocrinology clinic due to the presence of growth and/or development problems associated with this condition. Therefore, our sample may not be representative of the entire paediatric population with PP. This may also be the reason we found a higher prevalence in male patients compared to other published series. Longitudinal studies in boys with PP are scarce, but their findings evince that we need more prospective studies in this group to determine whether the prevalence of PP is changing in the population, along with its comorbidities and complications.
Since our study had a retrospective design, we were unable to analyse some relevant variables, such as testosterone levels in boys. Nevertheless, we believe that retrospective studies can contribute a considerable amount of information on certain diseases, and thus to improving our clinical practice so that we can offer the highest possible quality of care, as is the case of the one presented here.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Sancho Rodríguez ML, Bueno Lozano G, Labarta Aizpún JI, de Arriba Muñoz A. Evolución natural de la pubarquia precoz y posibles patologías asociadas. An Pediatr (Barc). 2018;89:238–245.
Previous presentation: This study was presented at the 39 Congress of the Sociedad Española de Endocrinología Pediátrica; May 10–12, 2017; Malaga, Spain.