Kawasaki disease is a self-limiting acute vasculitis that affects small and medium-sized vessels, and is the most common cause of acquired heart disease in children in our environment. Up to 25% of untreated patients develop coronary aneurysms. It is suspected that an infectious agent may be the trigger of the disease, but the causative agent is still unknown. Based on the previous evidence, recommendations are proposed for the diagnosis, treatment of acute disease, and the long-term management of these patients, in order to unify criteria. The diagnosis must be quick, based on easy-to-use algorithms and with the support of complementary tests. This document includes the indication of available imaging techniques, as well as the planning of cardiological examinations based on the initial involvement. Intravenous immunoglobulin is the basis of the initial treatment. The role of corticosteroids is still controversial, but there are studies that support its use as adjuvant treatment. A multidisciplinary working group has developed a scheme with different treatment guidelines depending on the risk factors at diagnosis, the patient's clinical situation, and response to previous treatment, including indications for thromboprophylaxis in patients with coronary involvement. The stratification of risk for long-term treatment is essential, as well as the recommendations on the procedures based on the initial cardiological involvement and its progression. Patients with coronary aneurysms require continuous and uninterrupted cardiological monitoring for life.
La enfermedad de Kawasaki es una vasculitis aguda autolimitada que afecta a vasos de pequeño y mediano calibre y es la causa más común de enfermedad cardiaca adquirida en niños en nuestro medio. Hasta un 25% de pacientes no tratados desarrollan aneurismas coronarios. Se sospecha que un agente infeccioso puede ser el desencadenante de la enfermedad, pero aún se desconoce el agente causal. En base a la evidencia previa, se proponen recomendaciones para el diagnóstico, tratamiento de la enfermedad aguda y manejo a largo plazo de estos pacientes, con el fin de unificar criterios. El diagnóstico debe ser rápido, basado en algoritmos de fácil manejo y con el apoyo de pruebas complementarias. Este documento recoge la indicación de las técnicas de imagen disponibles, así como la planificación de las revisiones cardiológicas en función de la afectación inicial. La inmunoglobulina intravenosa es la base del tratamiento inicial. El papel de los corticoides aún es controvertido, pero cada vez hay más estudios que avalan su uso como tratamiento adyuvante. Un equipo multidisciplinar ha elaborado un esquema con diferentes pautas de tratamiento en función de los factores de riesgo al diagnóstico, situación clínica del paciente y respuesta al tratamiento previo, incluyendo indicaciones sobre tromboprofilaxis en pacientes con afectación coronaria. La estratificación del riesgo para el tratamiento a largo plazo es esencial, así como las recomendaciones acerca del proceder en función de la afectación cardiológica inicial y su evolución. Los pacientes con aneurismas coronarios requieren un seguimiento cardiológico continuo e ininterrumpido de por vida.
Kawasaki disease (KD) is a self-limiting acute vasculitis that affects blood vessels of small and medium calibre. At present, it is the leading cause of acquired heart disease in children in developed countries and the second most frequent cause of vasculitis in children following Henoch-Schönlein purpura.1 Although the inflammatory process resolves spontaneously in most patients, up to 25% of untreated patients develop coronary artery complications, a proportion that decreases to approximately 4% in children treated with high-dose intravenous immunoglobulin (IVIG) through a mechanism that is yet unknown.2,3 It is suspected that an infectious agent may trigger the disease, but the causative agent has yet to be identified.4 Kawasaki disease is most prevalent in Asian countries, especially in Japan, where the incidence has been increasing to up to 265 cases per 100000 children aged less than 5 years5; in the United States, the incidence is approximately 25 per 100000 children aged less than 5 years,6 and in Europe it ranges between 5.4 and 15 per 100000 children aged less than 5 years.7,8 The overall incidence in Spain is unknown, but a recent study has described an incidence in Catalonia of 8 per 100000 children aged less than 5 years9 in the 2004–2013 period, similar to the incidence in the United Kingdom (8.4/100000)10.
Of all cases, 85% occur in children aged less than 5 years, with the incidence peaking between 18 and 24 months of life. Kawasaki disease is less frequent in infants aged less than 3 months or more than 5 years, although children in these age groups are at higher risk of developing coronary artery aneurisms. The male-to-female ratio is 1.5:1. There is evidence that KD is more common in winter and spring.11
The mortality of KD in Spain is not known, although mortality peaks between 15 and 45 days since onset of fever, when coronary artery vasculitis occurs concomitantly with significant elevation of the platelet count and a hypercoagulable state.
At present, a study known as KAWA-RACE is being conducted in Spain. It is a nationwide multicentre retrospective and prospective study of the epidemiological, clinical, laboratory and microbiological determinants of the response to treatment of KD and the risk of developing coronary aneurisms in patients aged less than 14 years. During the retrospective phase (2011–2016), the study included 625 patients. The results of this study, which have yet to be published at the time of this writing, will broaden our knowledge of KD in Spain.
Aetiology, pathogenesis and geneticsAlthough clinical and laboratory findings and the epidemiologic characteristics of the disease hint at an infectious cause or trigger, a specific aetiological agent has not been identified to date. Previous studies have also been unable to prove an association between the development of KD and exposure to certain drugs or the immune response to a superantigen.12
One of the theories that is currently most widely accepted is that KD is caused by an infectious agent that is inhaled and infects medium-size ciliated bronchial epithelial cells.13 Recent studies based on the analysis of the large epidemics of KD in Japan suggest that the causative agent could be an environmental agent borne by tropospheric winds, possibly a fungal toxin.14 At the same time, the high incidence in Asian communities and the increased risk of siblings of cases suggest that host genetic factors are important in the pathogenesis of KD. A few genome-wide association studies (GWAS) in patients with KD11 have been published that identified several loci implicated in inflammation, the immune response and cardiovascular involvement. Thus, a reasonable hypothesis is that KD is caused by an infectious agent yet to be identified that produces disease only in genetically predisposed individuals, especially those of Asian descent. Its low incidence in the first months of life and in adults suggests that this is an agent to which adults have developed immunity and newborns are passively protected against thanks to maternal antibodies.
Diagnosis of diseaseThe diagnosis is based on clinical criteria and is supported by serum values of inflammatory markers (Table 1). The identification of aneurisms in the coronary arteries or other locations confirms the diagnosis; however, coronary aneurisms are not usually detected until the first week from onset, so a normal echocardiographic examination early on does not rule out the diagnosis.11
Diagnostic criteria for classic Kawasaki disease.
| Fever of at least 5 days together with 4 of the 5 principal clinical criteria. In the presence of ≥ 4 principal clinical criteria, particularly when redness and swelling of the hands and feet are present, the diagnosis of KD can be made with 4 days of fever (in rare cases, experienced clinicians may establish the diagnosis with as few as 3 days of fever) Not all criteria need to be present at the same time. The feature may have resolved by the time of evaluation | |
| In cases where the clinical criteria for KD are not fully met, the presence of coronary abnormalities confirms the diagnosis | |
| Principal criterion | Description/comments |
| 1. Changes in the lips and/or oral mucosa | Erythema, cracking or bleeding of lips, strawberry tongue with prominent papillae, and/or erythema of oral or pharyngeal mucosa in the absence of compatible exudate or lesions |
| 2. Bulbar nonexudative conjunctival injection | Typically spares the limbus. Subconjunctival haemorrhage and punctate keratitis are occasionally observed |
| 3. Maculopapular eruption, diffuse erythroderma. Urticarial or micropustular eruptions may also be observed | Without vesicles/bullae, petechiae or scabs. Accentuation in the groin with early desquamation is a characteristic feature |
| 4. Palmar and plantar erythema and swelling during the acute stage and periungual peeling during subacute stage | The induration may be painful in some cases. Beau's lines (transverse grooves in nails) may be observed at 1–2 months |
| 5. Cervical adenopathy with diameter ≥1.5cm, usually unilateral | May be associated with retropharyngeal/parapharyngeal oedema |
| Features that support the diagnosis: elevation of inflammatory markers (CRP, ESR, PCT, leucocytosis with neutrophilia), hyponatremia and hypoalbuminemia, elevated transaminase levels and sterile pyuria. Thrombocytosis is a characteristic feature starting from the second week from onset. | |
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; PCT, procalcitonin.
The diagnosis of atypical or incomplete KD should be considered in patients with prolonged fever of unknown origin that meet fewer than 4 of the main clinical criteria and have compatible laboratory or echocardiographic findings (Fig. 1).11
Infants aged less than 6 months are more likely to have prolonged fever with no additional manifestations of KD and are at higher risk of developing coronary complications.
Cardiovascular involvement in Kawasaki diseaseCardiovascular manifestations and complications (Table 2) are the main cause of morbidity and mortality in KD, both in the acute stage and in the long term. Patients experience inflammation at the level of the pericardium, myocardium, endocardium (including the heart valves) and the coronary arteries.
Cardiovascular involvement in Kawasaki disease.
| Cardiovascular findings | ||
|---|---|---|
| In the acute stage, features may include a hyperdynamic precordium and tachycardia. Patients may present with a gallop rhythm suggestive of diastolic dysfunction secondary to myocardial inflammation and oedema. The presence of a pericardial rub or signs of pericardial tamponade is rare (if there is pericardial effusion, it is usually small). A pansystolic murmur may be heard on auscultation in children with mitral regurgitation. A diastolic murmur associated with aortic regurgitation is rare. Signs and symptoms of myocardial ischaemia are atypical and nonspecific in children. | ||
| Lesion | Incidence | Characteristics |
| Coronary abnormalities | Up to 25% in untreated patients. The risk drops to <5% with IVIG treatment. Retrospective phase of KAWARACE study: incidence of coronary aneurisms, 9.6% | They are a primary determinant of prognosis. In 50% of patients, and depending on the size, they resolve within 2 years. Patients with large/giant aneurisms do not have cardiovascular manifestations except for development of myocardial ischaemia due to severe changes in blood flow or thrombosis. The risk of coronary aneurism rupture is very low. It may occur during the acute phase due to rapid growth of the aneurism |
| Myocarditis | Nearly universal (case series that used labelled WBC heart scans found myocardial involvement in 50%-70% of cases) | It develops prior to coronary artery abnormalities and without concurrent ischaemic damage. It does not lead to cellular disruption or myocyte necrosis: the myocarditis is transient and responds quickly to anti-inflammatory treatment. Assessment of ventricular systolic and diastolic function is necessary in all patients |
| Cardiogenic shock | It is the initial presentation in approximately 5% of patients | Thrombocytopaenia and coagulopathy are common in these cases. Patients with this presentation are at higher risk of IVIG resistance, coronary involvement and prolonged myocardial dysfunction |
| Valvulitis | ||
| Mitral regurgitation | In the acute stage in up to 25% of patients. | In the early course of KD, it is usually of moderate severity, and it does not appear to persist on follow-up. It has been correlated with other laboratory markers of inflammation Its development seems to be associated with the pancarditis or the shared inflammatory process that takes place during the acute stage of KD. |
| Aortic regurgitation | Much less frequent (1%) | It is associated with aortic root dilation in the early course of illness, and has been reported in 10% of patients during the acute stage of KD. Its presence has been associated with coronary artery dilation |
| Pericarditis | 6–24% of patients | In most patients it is limited to the acute stage of disease, and it is usually mild and transient. |
Recent microscopy studies have identified 3 vasculopathic processes: the first is a self-limited process that ends about 2 weeks from onset, a necrotising arteritis produced by infiltration of activated neutrophils into the adventitia that causes aneurisms. The second process is a subacute/chronic vasculitis with infiltration of lymphocytes, plasma cells, eosinophils and macrophages that starts 2 weeks from onset and may continue for months or even years in some patients. The third is luminal myofibroblastic proliferation of the medial smooth muscle cells that starts in the first 2 weeks and persists for months or years and can eventually cause arterial stenosis.15
Diagnosis of cardiovascular involvementEchocardiogramEchocardiography is the gold-standard imaging test for the evaluation of the coronary arteries, ventricular function, pericardial/pleural effusion and valve regurgitation in the acute stage of KD. Table 3 proposes a protocol for cardiovascular assessment in KD that takes into account the abnormalities described most frequently in the current literature.11,16
Evaluation of cardiovascular abnormalities in Kawasaki disease.
| The cardiovascular evaluation should include: history taking, physical examination, electrocardiogram and echocardiogram | |||||
|---|---|---|---|---|---|
| Abnormality | Assessment | ||||
| Coronary abnormalities | Echocardiogram: Use the highest-frequency transducer possible, reduce the window to the region under study, focusing on the area of interest. Compress 50–60 and gain 60–65%. Measure inner edges of coronary arteries excluding points of branching. Coronary abnormalities typically start to develop in the proximal segments and extend distally 1. Size (z-score): | ||||
| No involvement | Dilation/ectasia | Small aneurisms | Midsized aneurisms | Giant aneurisms | |
| z-score: <2 | z-score ≥2 and <2.5 | z-score ≥2.5 and <5 | z-score ≥5 and <10 and diameter <8mm | z-score ≥10 and/or diameter ≥8mm | |
| 2. Location: identify the affected coronary artery and segment (proximal, medial or distal) Echocardiographic views: - Left main coronary artery: precordial short axis at level of aortic valve; precordial long axis of left ventricle (superior tangential); subcostal ventricular long axis - Left anterior descending: precordial short axis at level of aortic valve; precordial superior tangential long axis of left ventricle; precordial short axis of left ventricle - Circumflex branch: precordial short axis at level of aortic valve, apical 4-chamber - Right coronary artery: precordial short axis at level of aortic valve; precordial long axis (inferior tangential) of left ventricle; subcostal short axis at level of atrioventricular groove; subcostal coronal projection of right ventricular outflow tract - Posterior descending: apical 4-chamber (inferior), precordial long axis (inferior tangential), subcostal (inferior) 3. Morphology: - Saccular: when axial and lateral diameters are nearly equal - Fusiform: when coronary dilation is symmetrical with progressive proximal and distal tapering of luminal diameter | |||||
| Electrocardiogram: Depending on the involvement, the infarction may be: - Subepicardial: T-wave changes - Subendocardial: ST changes - Transmural: pathologic Q waves During acute illness, it can be used to monitor the changes that usually occur in this type of lesions Approximate localisation of coronary artery involvement during acute illness: | |||||
| Proximal ADCA | Proximal to first septal branch | ST elevation in V1-V6, aVL, RBBB | |||
| Medial ADCA | Distal to first septal branch and proximal to diagonal branch | ST elevation in V1-V4, aVL | |||
| Distal ADCA | Distal to diagonal branch | ST elevation V1-V4, or I, aVL | |||
| RCA or circumflex artery | Moderate or severe inferior infarction (posterior, lateral, RV | ST elevation in II, III and aVF | |||
| RCA or circumflex artery | Strictly inferior infarction | ST elevation in II, III and aVF | |||
| Myocarditis Cardiogenic shock | Echocardiogram: diastolic and systolic function, overall and by segment: - Ejection fraction (EF): calculate with Teicholz formula in the long axis and with Simpson's rule in 4-chamber view. Dysfunction if EF<60% - Tissue doppler (mitral valve, tricuspid valve and interventricular septum) in 4 chambers: S, E′, A′ waves, isovolumic contraction time, isovolumic relaxation time and ejection time Electrocardiogram: rule out sinus node and atrioventricular abnormalities, prolonged PR interval, pathologic Q waves, prolonged QT interval, low-voltage waves, ST-T abnormalities, arrhythmias and repolarization abnormalities | ||||
| Mitral regurgitation | Echocardiogram: assess presence or absence of valvular regurgitation and its severity by colour flow and pulsed-wave Doppler in the apical 4-chamber view Electrocardiogram: rule out left atrial enlargement in severe cases | ||||
| Aortic regurgitation | Echocardiogram: assess for aortic root dilation in the precordial long axis view, and assess for valvular regurgitation and its severity by colour flow and pulsed-wave Doppler in the precordial long axis and apical 5-chamber views Electrocardiogram: in cases of moderate to severe regurgitation, assess for left ventricular hypertrophy, and in cases of advanced disease, ST-T wave abnormalities due to ischaemia | ||||
| Pericarditis | Echocardiogram: assess presence and severity of pericardial effusion in apical and subcostal 4-chamber views Electrocardiogram: Stage I: elevation of ST segment with positive T wave and PR or PQ interval depression. Stage II: flattening of ST segment and T wave. Stage III: T wave inversion. Stage IV: normalised T wave | ||||
ADCA, anterior descending coronary artery; RBBB, right bundle branch block; RCA, right coronary artery; RV, right ventricle.
Electrocardiogram frontal plane leads: I, II, III, aVR, aVF, aVL. Precordial leads: V1-V8.
In general, routine use of transoesophageal echocardiography, coronary angiography, coronary magnetic resonance imaging or computerised axial tomography is not indicated for diagnosis or management of KD in the acute stage.
In cases where there are limitations to the echocardiographic evaluation, for instance due to the presence of thrombi or stenosis, or in older children and/or adolescents in which the echocardiographic resolution is not adequate, performance of more advanced imaging tests could be indicated, especially in patients with serious abnormalities in the proximal part of the coronary arteries in whom it is necessary to examine the distal segments to make treatment decisions (Table 4).17,18
Criteria for performance of imaging tests other than echocardiography.
| CT coronary angiogram |
| Routine use is not indicated In case of giant aneurisms located in proximal/distal regions of the arteries (visible on echocardiography), it is used for two purposes: To define the size of aneurysms at this level and assess distal coronary segments To assess the presence/absence of thrombi/stenosis in coronary arteries Preferably with a prospective ECG-triggered protocol, using the step-and-shoot technique with administration of beta blockers to achieve a heart rate <70bpm. Dose, 1.5-3mSv. Patients aged < 7-8 years may require sedation |
| Cardiac MRI |
| Routine use is not indicated In patients with coronary artery involvement detected by echocardiography classified at risk level ≥3 (Table 6) and/or with suspected associated myocarditis (on echocardiography), it is useful to assess overall/segmental systolic function and the myocardium (perfusion MRI and delayed enhancement MRI) Cardiac MRI is not the gold standard to assess coronary anatomy. However, it may be used for this purpose if it is already being performed for another reason Comparison with CT: this technique does not involve exposure to radiation, but requires longer image acquisition times (sedation in many cases) and offers lower resolution of coronary anatomy imaging |
| Cardiac catheterization |
| - Not recommended in acute stage of disease - Unnecessary in patients without coronary abnormalities on echocardiography or with ectasias - In patients with a single small or medium-sized aneurism, it should only be performed if the results of the evaluation of myocardial ischaemia were positive or the findings of imaging tests are compatible with stenosis - In patients with a giant aneurism or several small/medium-sized aneurisms, perform if tests to assess for myocardial ischaemia are positive or there are clinical or echocardiographic changes suggestive of acute coronary disease |
The inflammation at the cardiac level that occurs in the acute stage of KD involves mainly the coronary arteries, although there may also be clinical or subclinical myocardial inflammation that produces electrocardiographic changes indicative of myocardial and/or coronary artery involvement (Table 3).
TreatmentTreatment of KD in the acute stageThe first-line medical treatment in KD is intravenous immunoglobulin (IVIG) infusion therapy.11 There is ample evidence of the efficacy of IVIG during the acute stage in reducing the incidence of coronary aneurisms.19–21 Gamma globulin is a biologic drug consisting of a preparation with a high concentration of immunoglobulin G (≥95%) and other human immunoglobulins. Its mechanism of action remains unknown. It should be administered as early as possible within 10 days from onset, or even later in case of persistence of the fever of unknown origin, persistence of inflammatory activity as evinced by the elevation of CRP levels or the ESR, or presence of coronary aneurisms. The standard regimen is a single infusion of 2g/kg of IVIG (Table 5).20,22 Concomitant treatment with acetylsalicylic acid (ASA)23 should be initiated, at a moderate dose (30–50mg/kg/day every 6h, administered orally) until the patient has been afebrile for 48–72h, subsequently adjusted to a lower dose to achieve an antiplatelet effect (3–5mg/kg/day in a single dose, administered orally) to be maintained through 6–8 weeks from onset and normalisation of the platelet count, acute phase reactant levels and echocardiographic features. In spite of its anti-inflammatory activity, it appears that treatment with ASA does not reduce the incidence of coronary aneurisms, however, in the studies that demonstrated the efficacy of IVIG it was used as adjuvant treatment, so ASA is traditionally associated with IVIG therapy.24
Drugs used in the management of Kawasaki disease.
| Treatment of acute illness | |||
|---|---|---|---|
| First-line drugs | |||
| Drug | Dose | Adverse reactions/precautions | Pharmaceutical form |
| IVIG | Single dose: 2g/kg IV | Associated with a high infusion rate at beginning of treatment, in 5–15%: fever, chills, headache, myalgia, nausea, vomiting. During or up to 1–2 days after infusion. An anaphylactic reaction may occur in children with IgA deficiency due to the development by the recipient of anti-IgA antibodies during previous treatment with IVIG. In these cases, select the preparation with the least amount of IgA of those available. Measles, mumps, rubella and varicella immunizations should be deferred for 11 months after IVIG administration Infusion rate: always monitor the first infusion of IVIG for the first 30min. Initial rate for 5% solution: 0.5mL/kg/h in the first 30min. For 10% solution: 0.25ml/kg/h. The rate can be increased progressively until reaching the maximum specified by the manufacturer (consult summary of product characteristics). Usually given over 12h. The first time IVIG is given to a patient it must be prepared as a 5% solution | Vials, 50mg/mL: 0.5g, 1g, 2.5g, 5g, 10g, 20g. Vials, 100mg/mL: 1g, 2g, 2.5g, 5g, 10g, 20g. Vials, lyophilised powder: 2.5g, 5g, 10g |
| ASA | Anti-inflammatory: 30–50mg/kg/day every 6h, p.o. | At anti-inflammatory doses, it may cause mild chronic salicylate intoxication, which is characterised by tinnitus and hearing loss. Discontinue treatment if these symptoms appear. Hypoprothrombinaemia, rhinitis, paroxysmal bronchospasm, gastrointestinal changes and bleeding | Tablets: 100, 125, 150, 250, 300 and 500mg |
| Antiaggregant: 3–5mg/kg/day in 1 dose, p.o. | Concomitant administration of ibuprofen antagonises the irreversible platelet inhibition effect of ASA, so ibuprofen is contraindicated in these patients Use caution if patient has an active varicella or influenza infection on account of the risk of Reye syndrome. If the patient has an influenza infection in the acute stage of KD, aspirin must be avoided (use paracetamol for fever reduction and another antiplatelet agent, such as clopidogrel, for at least 2 weeks). Children aged > 6 months should receive the inactivated influenza vaccine. In case of exposure to varicella, ASA must be discontinued and replaced by a different antiplatelet agent. After vaccination against varicella, consider switching ASA to an alternative antiplatelet agent for 6 weeks. However, there is no evidence of an association of ASA with Reye syndrome at doses of 3–5mg/kg/d | ||
| Corticoids | Various regimens: - Methylprednisolone, 30mg–1/kg/day IV for 3 days, followed by methylprednisolone, prednisolone or prednisone 2mg/kg/day IV or p.o. with gradual taper based on patient evolution (Fig. 2) - Methylprednisolone 2mg/kg/day IV until fever resolves and CRP levels decrease, with gradual taper based on patient evolution (Fig. 2) | Acne, hypokalaemia, fluid retention, alkalosis, weakness, myopathy with muscle atrophy, cataracts, raised intracranial pressure, high blood pressure, osteoporosis, Cushing syndrome, adrenal suppression, peptic ulcer, glucose intolerance, hirsutism, amenorrhoea, infection, delayed growth | Methylprednisolone: Vials: 8, 20, 40, 125, 250, 500, 1000mg. Tablets: 4, 16, 40mg. Prednisolone: suspension, 7mg/mL Prednisone: tablets, 2.5, 5, 10, 30, 50mg |
| Second-line drugs (supervision by an expert is recommended) | |||
| Infliximab | 6mg/kg IV over 2h 1–2 doses (if 2 doses, administer 1 dose/week) | Very frequent (>1/10): risk of infection, headache, nauseas, abdominal pain. Frequent (1/10–1/100): neoplasia, neutropenia, leukopenia, anaemia, lymphadenopathies, respiratory allergy symptoms, depression, insomnia, conjunctivitis, hypotension, hypertension, ecchymosis, hot flashes, facial redness, liver failure, urticaria, eruption, pruritus, hyperhidrosis, dry skin, fungal dermatitis, eczema, alopecia, arthralgia, myalgia, back pain | Powder for concentrate for solution for infusion: 100mg |
| Anakinra | 2–6mg/kg/day subcut, 15 days | Infections, neutropenia, thrombocytopenia, headache, local reaction at injection site, hypercholesterolaemia | 100mg solution for injection in pre-filled syringe |
| Etanercept | 0.8mg/kg/dose IV weekly (3 doses) | Local reaction at injection site, risk of infection, allergic reaction, development of autoantibodies, fever, pruritus | 10mg powder and solvent for solution for injection for paediatric use |
| Ciclosporin | 3mg/kg/day IV every 12h 4–8mg/kg/day p.o. every 12h | Very frequent (>1/10): hyperlipidaemia, hypercholesterolaemia, high blood pressure, tremors, headache, renal failure Frequent (1/10–1/100): skin rash, oedema, convulsive seizures, hirsutism, hyperkalaemia, hypomagnesemia, hyperuricemia, diabetes, cholestasis, gingival enlargement, lymphoproliferative syndromes, liver and gastrointestinal changes | Soft capsules: 25, 50, 100mg Solution, 100mg/mL. Ampoules: 50g/mL, 250mg/5mL |
| Cyclophosphamide | 2mg/kg/day IV single infusion | Dose-dependent myelosuppression, haemorrhagic cystitis, nausea and vomiting, reversible alopecia, syndrome of inappropriate antidiuretic hormone secretion, renal fibrosis, sterility, aspermia or azoospermia and amenorrhoea | Powder for injectable solution: 50, 100, 200mg. |
| Antiplatelet, anticoagulant and fibrinolytic drugs | |||
| Dipyridamole | Antiplatelet dose: 3–6mg/kg/day every 8h p.o. Administer with water or milk on empty stomach, 1h before or 2h after eating | Vasodilatation, antihypertensor, dizziness, headache (dose-dependent), exanthema, pruritus, gastrointestinal changes Increased risk of haemorrhage in association with use of anticoagulant drugs or drugs with an antiplatelet effect (omega 3 fatty acids and vitamin E, NSAIDs and valproic acid). Increases the levels and amplifies effect of: adenosine, and its β-blocker effect (bradycardia) Dampens effect of cholinesterase inhibitors | Tablets: 100mg Compounded preparation: 10mg/mL oral suspension |
| Clopidogrel | - Newborns and <2 years: 0.2mg/kg/day very 24h p.o. - >2 years: 1mg/kg/day p.o. titrated to maximum response. 75mg Administer with or without food | Pruritus, gastrointestinal bleeding, epistaxis Increases risk of haemorrhage in combination with drugs that cause changes in haemostasis Reduced effect in CYP2C19 poor metabolizers | Tablets: 75mg, 300mg Compounded preparation: 5mg/mL oral suspension |
| Abciximab | Loading dose: 0.25mg/kg as IV bolus given over 10–60min, followed by continuous infusion at 0.125μg/kg/min (maximum 10μg/min over 12h IV) Concentration of solution for adults: 28.8μg/mL or 36μg/mL diluted in dextrose saline or physiological saline | Risk of haemorrhage that increases with other drugs or factors that affect haemostasis (4–17%) Other (>10%): hypotension, chest pain, lumbar pain, nausea. May cause hypersensitivity reactions. Administer through access in a compressible site. Do not administer in case of recent history (<6 weeks) of abdominal bleeding, surgery or major injury, stroke, thrombocytopenia (<100000/μL). Monitor platelet count at 2–4h from initial bolus and at 12h from initiation. Onset of action at 10min, duration of antiplatelet effect of 72h up to 7 days | Solution for injection and infusion, 2mg/mL |
| Low molecular weight heparin (enoxaparin sodium) | In paediatrics, do not administer every 24h due to faster clearance of drug - <12 months: Treatment: 3mg/kg/day subcut every 12h Prophylaxis: 1.5mg/kg/day subcut every 12h. - Children and adolescents: Treatment: 2mg/kg/day subcut every 12h Prophylaxis: 1mg/kg/day every 12h | Major adverse reactions: anaphylactic shock, haemorrhage, thrombocytopenia, thrombosis Treatment dose: 0.5 – 1U/mL Prophylaxis dose: 0.1– 0.3U/mL Adjust subsequent doses based on anti-factor Xa activity and specific nomogram. Adjust dosage in case of severe renal failure Risk of hyperkalaemia | Solution in pre-filled syringes, 100mg/mL Different preparations: 10000IU/mL and 15000IU/mL |
| Unfractionated heparin sodium | Loading dose: 75U/kg as IV bolus over 10min Maintenance: <1 year: 28IU/kg/h IV >1 year: 20IU/kg/h IV Measure aPTT at 6h, target: 60–85s | High risk of haemorrhage. Difficult management. Limited experience in paediatric use High variability between patients | |
| Acenocoumarol | First dose: - Newborns: 0.2mg/kg p.o. - <1 year: 0.1mg/kg/day p.o. - 1–5 years: 0.06mg/kg day p.o. Adjust based on INR value (target, 2–3) Peak action at 36–48h | Increased risk of haemorrhage. Discontinue if INR > 4 Effects may be affected by dietary intake of vitamin K | Tablets: 1 and 4mg. |
| Alteplase | IV thrombolysis: Standard dose: 0.5mg/kg/h (0.1–0.6mg/kg/h for 6h. Low dose: 0.03–0.06mg/kg/h for 12–48h Maximum 2mg/h | High risk of haemorrhage. Cholesterol crystal embolisation. Lingual angioedema | Powder and solvent for solution for injection and infusion: vials of 10mg, 20mg and 50mg to prepare 1mg/mL solution |
| Urokinase | IV thrombolysis: 1.0–1.6×104U/kg Over 30–60min Intracoronary thrombolysis: 0.4×104U/kg over 10min Can be repeated up to 4 times | High risk of haemorrhage | Powder and solvent for solution for injection and infusion: vials of 100000IU and 250000IU to prepare 50000IU/mL solution |
Approximately 10–20% of patients with KD have persistent fever 36h after treatment with IVIG and ASA.25 In these patients, inflammation and the risk of coronary damage therefore persist.
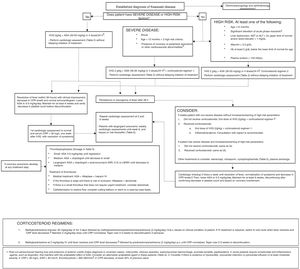
The use of corticosteroids as primary adjuvant treatment remains controversial, but a growing number of studies support it.26,27
In a study in Japanese patients with KD with a score indicative of high risk of resistance to primary treatment with IVIG therapy, the addition of corticosteroids to IVIG and ASA was associated with a decrease in inflammation, improved coronary outcomes and a reduced duration of symptoms,26 and the Japanese Circulation Society guidelines include steroid therapy as a first-line adjuvant treatment in these cases.16
Two recent systematic reviews with meta-analyses have reported that corticosteroid therapy has proven effective in the prevention of coronary lesions, although the source studies were mainly conducted in Japanese patients.28,29 The Japanese scoring systems have not been validated in populations in our region or in the United States.30 The American Heart Association considers the use of a long course of steroids concomitant with IVIG therapy in patients with risk factors for IVIG resistance, and administration of high-dose methylprednisolone boluses as adjuvant treatment in patients that do not respond to the initial or repeated dose of IVIG or concomitantly to the second dose of IVIG in patients that do not respond to initial IVIG therapy combined with a long course and taper of oral steroids.11
Corticosteroids may be considered for prophylaxis in children with severe KD and as rescue therapy in patients that do not respond to initial treatment. However, the routine use of corticosteroid therapy in all patients with KD needs to be supported by further research, especially outside Japan.
There is evidence that biological therapy with infliximab, a monoclonal antibody specific for tumour necrosis factor alpha (TNFα), is efficacious in reducing inflammation, but not in suppressing vasculitis.31 Its use as adjuvant therapy to first-line treatment seems safe, but does not improve coronary outcomes.
The current evidence is not conclusive as regards the management of patients resistant to the initial IVIG dose. Many experts recommend a second dose of IVIG, although clinical trials have yet to be performed to assess the efficacy of this approach.3 Corticosteroid therapy has also been used as second-line treatment in these patients, as noted above. It appears that long courses of oral corticosteroids may suppress vascular inflammation, but there have been no clinical trials comparing different corticosteroid regimens.
Two retrospective studies and one randomised multicentre study have compared the use of infliximab in patients resistant to IVIG to administration of a second dose of IVIG.32 Treatment with infliximab was associated with a reduction in length of stay and in the duration of fever, but not in the incidence of cardiovascular sequelae or adverse events. There are 3 published case reports describing the successful use of anakinra, an interleukin-1 (IL-1) receptor antagonist, in patients with KD highly refractory to conventional treatment, and clinical trials that evaluate its efficacy in acute KD are currently underway.33–36 Anakinra has been associated with a decrease in the duration of fever and serum marker levels as well as improved short-term coronary outcomes, so its use should be considered for rescue of patients that do not respond to conventional treatment.
Ciclosporin seems to reduce the length of stay and duration of fever, but not the incidence of coronary complications. A clinical trial of ciclosporin in combination with IVIG is currently in progress.37 Cyclophosphamide, which is widely used to treat other types of vasculitis, should be reserved for very severe cases on account of its side effects, as should plasma exchange.
There are cases where diagnosis is delayed (past 10 days from onset) and the fever and elevation of acute phase reactants have already resolved. In these cases, treatment with low-dose ASA for an antiplatelet effect (3–5mg/kg/day) should be initiated and maintained until the end of the acute stage of disease (6–8 weeks), verifying the normalisation of echocardiographic features and platelet count before discontinuation.
Based on the current evidence, this consensus document proposes different treatment regimens according to the presence of risk factors at diagnosis, the clinical condition of the patient and the response to previous conventional and steroid treatment (Fig. 2).
Note: our working group recommends consultation with an expert/team of experts to make treatment decisions if there is any uncertainty or in particularly complex cases.
Treatment of cardiomyopathy/shock during acute stageMyocardial function usually recovers after treatment with IVIG, as the latter curbs inflammation and systemic manifestations. In cases with mild haemodynamic instability, patients usually respond to treatment with diuretic and vasopressor agents. Shock may have a cardiogenic, distributive or mixed cause, with a pathophysiology similar to that of septic shock, with vasodilation produced by inflammatory factors, absolute and relative hypovolaemia and myocardial dysfunction. In these cases, treatment with IVIG should be combined with the use of inotropic and vasopressor agents (dobutamine, epinephrine, norepinephrine and dopamine).11
Prevention and treatment of thrombosis in patients with coronary aneurismsIn addition to rupture of a coronary artery aneurism, which is a rare occurrence, thrombotic occlusion of a coronary aneurism followed by secondary myocardial infarction is the most frequent complication in the acute stage of KD.
Coronary thrombosis should be suspected in patients with rapid deterioration of ventricular function or electrocardiographic changes. In case of coronary aneurisms with a progressive increase in diameter, the use of antiplatelet agents should be considered (for example, adding clopidogrel to ASA), as inadequate thromboprophylaxis in patients with coronary abnormalities is the strongest predictor of a poor outcome during the acute stage of disease.
The management in this section has been extrapolated from clinical practice in adults with coronary or cerebrovascular disease (Tables 4 and 5).
Risk stratification and follow-upPatients with KD are stratified into different groups according to coronary involvement regardless of the stage of disease. In addition to the size of aneurisms, the risk factors for ischaemia that need to be considered are: extent of maximal involvement, distal location, absence of collateral vessels, obstruction, prior history of thrombosis, acute myocardial infarction (AMI), revascularisation or presence of ventricular dysfunction. The greater the extent of coronary involvement, the higher the risk of developing ischaemia, so the management and follow-up will vary between groups (Table 6). The long-term management protocol should be initiated at the end of the acute stage (4–6 weeks) when coronary artery luminal diameters are no longer enlarging.
Cardiology followup based on risk in patients with Kawasaki disease.
| Risk level | Cardiology followupa | Followup tests | Pharmacological treatment | Nonpharmacological treatment and physical activity recommendations |
|---|---|---|---|---|
| 1. No coronary involvement at any point (z-score always <2) | Not needed. Discharge at 6 weeks from onset. Consider checkup at 12 months | - Not needed. - Prevention of cardiovascular risk factorsb | ASA (3–5mg/kg) until 6 weeks from onset of KD No need of ASA thereafter | Restriction of physical activity is not recommended beyond 6–8 weeks |
| 2. Dilation (z-score always 2–2.5) | Discharge at 12 months (If dilation persists, checkup every 2–5 years) | Consider continuing ASA (3–5mg/kg) if dilation persists | ||
| 3. Small aneurism (z-score ≥ 2.5 to <5) | ||||
| 3.1 Persistent | 6 and 12 months (first year), once a year thereafter | - Cardiac stress testc every 2–3 years or if patient exhibits symptoms of ischaemia or signs of ventricular dysfunction - Consider angiographyd every 3–5 years - Prevention of cardiovascular risk factorsb | ASA (3–5mg/kg) until aneurisms regress Consider statins | < 11 years: restriction of physical activity is not recommended beyond 6–8 weeks > 11 years: consider restriction of physical activity based on results of cardiac stress test and functional testing |
| 3.2 Decreased to dilation or normal luminal dimension | 1–3 years | - Cardiac stress testc every 3–5 years or if patient exhibits symptoms of ischaemia or signs of ventricular dysfunction - Consider angiographyd in case of inducible ischaemia - Prevention of cardiovascular risk factorsb | ||
| 4. Medium aneurism (z-score ≥ 5 to <10 and absolute dimension < 8mm) | ||||
| 4.1 Persistent | 3–6-12 months (first year). 6–12 months | - Cardiac stress testc every 1–3 years or if patient exhibits symptoms of ischaemia or signs of ventricular dysfunction - Consider angiographyd every 2–5 years - Prevention of cardiovascular risk factorsb | ASA (3–5mg/kg) Consider anticoagulation therapy (Acenocoumarol/LMWH) or dual antiplatelet therapy (clopidogrel) if aneurisms persist Consider dual antiplatelet therapy (add clopidogrel) if aneurisms regress Consider statins | <11 years: restriction of physical activity is not recommended beyond 6–8 weeks > 11 years: consider restriction of physical activity based on results of cardiac stress test and functional testing If anticoagulation is used, avoid contact sports |
| 4.2 Decreased to small aneurism | 1 year | - Cardiac stress testc every 2–3 years or if patient exhibits symptoms of ischaemia or signs of ventricular dysfunction - Consider angiographyd every 3–5 years - Prevention of cardiovascular risk factorsb | ||
| 4.3 Decreased to dilation or normal luminal dimension | 1–2 years | - Cardiac stress testc every 2–4 years or if patient exhibits symptoms of ischaemia or signs of ventricular dysfunction - Angiographyd in case of inducible ischaemia - Prevention of cardiovascular risk factorsb | ||
| 5. Giant aneurism (z-score ≥ 10 and/or ≥ 8mm) | ||||
| 5.1. Persistent | 1–2-3–6-9–12 months (first year). 3–6 months | - Cardiac stress testc every 6–12 months or if patient exhibits symptoms of ischaemia or signs of ventricular dysfunction - Consider angiographyd at 6–12 months and every 1–5 years - Prevention of cardiovascular risk factorsb | ASA (3–5mg/kg). Anticoagulation with acenocoumarol or LMWH if aneurism persists or decreases to medium size Consider if aneurism decreases to small size, discontinue if aneurism regresses Consider dual antiplatelet therapy (ASA+clopidogrel), with anticoagulation if aneurism persists, or in place of anticoagulation if aneurism decreases or regresses Consider treatment with beta blockers Consider statins | Restriction of physical activity based on results of cardiac stress test and functional testing If anticoagulation is used, avoid contact sports |
| 5.2. Decreased to medium aneurism | 6–12 months | - Cardiac stress testc every year or if patient exhibits symptoms of ischaemia or signs of ventricular dysfunction - Consider angiographyd every 2–5 years - Prevention of cardiovascular risk factorsb | ||
| 5.3 Decreased to small aneurism | 6–12 months | - Cardiac stress testc every 1–2 years or if patient exhibits symptoms of ischaemia or signs of ventricular dysfunction - Consider angiographyd every 2–5 years - Prevention of cardiovascular risk factorsb | ||
| 5.4. Decreased to dilation or normal luminal diameter | 1–2 years | - Consider cardiac stress testc every 2–5 years or if patient exhibits symptoms of ischaemia or signs of ventricular dysfunction - Angiographyd in case of inducible ischaemia - prevention of cardiovascular risk factorsb | ||
| Stenosise and/or thrombosis | Every 6 months | - Cardiac stress testc yearly - Angiography at intake and during followup based on patient progress | Anticoagulants Beta blockers | Cardiac catheterization in cases with severe stenosis in 5 coronary arteries Coronary artery bypass in cases with severe occlusion in left coronary artery or involvement of 2 or 3 vessels, as long as there is viable myocardium |
The cardiologic assessment includes history and physical examination, electrocardiogram and echocardiogram (the latter is not essential in patients with normalisation of the coronary arteries, unless they exhibit symptoms of ischaemia, ventricular dysfunction or inducible ischaemia).
Primary prevention of cardiovascular risk factors includes: measurement of blood pressure, monitoring of body mass index (BMI) and waist circumference, education on healthy dietary habits and prevention of smoking and sedentary lifestyles. In patients with a history of aneurism, performance of a lipid profile every 5 years. Follow-up may be carried out by the primary care paediatrician.
Cardiac stress tests: stress echocardiography, stress magnetic resonance imaging (MRI), stress nuclear medicine (NM) perfusion imaging or positron-emission tomography (PET). The choice of method will be made by each facility based on its experience and minimising patient risk. In children aged <6 years who are asymptomatic, have no symptoms of ischaemia or signs of ventricular dysfunction, consider non-invasive coronary artery imaging at rest.
Coronary angiography can be performed through non-invasive methods (PET, MRI, CT) or invasively (catheterization). In case of inducible ischaemia, catheterization is the method of choice. Coronary angiography should not be performed during the acute stage: defer until at least 6 months from diagnosis.
The cardiovascular risk of patients without aneurisms is similar to that of the general population, so these patients may be discharged from follow-up in the cardiology department after verifying the normalisation of coronary artery features, with an emphasis on the evaluation of cardiovascular risk factors.2
In cases where aneurisms develop, the latter resolve in the first 3 months in 15% of patients, and there is regression from initial measurement in most patients within the next 2 years, depending on the extent of involvement.38 Despite regression, the aneurismal area may narrow progressively as a result of luminal myofibroblastic proliferation. For this reason, patients that develop aneurisms in the acute stage of KD require long-term cardiologic follow-up, regardless of regression.
Patients with severe coronary artery involvement do not usually develop cardiologic symptoms unless they suffer myocardial ischaemia secondary to obstruction and thrombosis.
The signs and symptoms of AMI may be atypical and nonspecific in children, especially in infants. Few cases of myocardial ischaemia have been reported in children due to the development of collateral vessels, and they have been associated with the rupture of aneurisms during acute illness due to their rapid growth.
In cases where there is evidence of inducible myocardial ischaemia, performance of invasive angiography is recommended to assess for coronary stenosis, even if the patient is asymptomatic.
Adult patients with Kawasaki diseaseAcute-stage KD does not usually occur in adults. Patients with KD have usually been discharged or are those that have developed sequelae. Their follow-up is planned according to the presence and severity of coronary involvement, past and present, focusing on abnormalities in the coronary arteries, valve function and myocardial abnormalities (function, perfusion and presence of scar tissue) (Table 6).
Coronary aneurysms are located at the epicardial level, and the most frequent locations are the proximal segments of the anterior descending artery and right coronary artery, followed by the left main trunk and the circumflex artery, distal segments of the right coronary artery and the posterior descending artery, with a predominance of involvement at branching points.
The long-term mortality of Japanese patients with a history of KD and cardiovascular sequelae is higher compared to the general population.39 Recent studies have suggested a high incidence of adverse events associated with KD in young adults. In the United States, 5% AMIs in individuals aged less than 40 years occur in patients with a known or suspected history of KD (1.5% and 3.5%, respectively).40 In Japan, up to 9% of AMIs and sudden cardiac deaths in young adults are attributable to a previous history of KD.16
The AHA recommends transitioning to adult cardiology care at age 18–21 years.11 Adult cardiologists must be aware of this growing cohort of young adults at risk of cardiovascular sequelae from their childhood disease, which makes the collaboration between paediatric and adult cardiologists essential.
Note: to participate in the KAWA-RACE study, contact kawasaki.kawarace@gmail.com.
Conflict of interestThe authors have no conflicts of interest to declare.
We thank Dr Masaru Miura, adjunct physician of the Cardiology Department of the Tokyo Metropolitan Children's Medical Center, for his invaluable help in the drafting and editing of this article.
Members of the Working Group on Clinical Cardiology of the Sociedad Española de Cardiología Pediátrica y Cardiopatías Congénitas (Spanish Society of Paediatric Cardiology and Congenital Heart Defects [SECPCC]):
Appendix A lists the names of all the authors of the article.
Please cite this article as: Tascón AB, Malfaz FC, Sombrero HR, Fernández-Cooke E, Sánchez-Manubens J, Pérez-Lescure Picarzo J, et al. Consenso nacional sobre diagnóstico, tratamiento y seguimiento cardiológico de la enfermedad de Kawasaki. An Pediatr (Barc). 2018;89:188.
This document is endorsed by the following scientific societies: Asociación Española de Pediatría (AEP); Sociedad Española de Cardiología (SEC); Sociedad Española de Infectología Pediátrica (SEIP); Sociedad Española de Reumatología Pediátrica (SERPE); Asociación Española de Pediatría de Atención Primaria (AEPap).