Immune thrombocytopaenia (ITP) is a relatively common disorder in childhood. Although it usually achieves spontaneous remission at this age, the management of persistent or chronic ITP in children is still controversial. The aim of this article is to address current controversies related to the treatment of persistent, chronic, and refractory ITP in children, including the role of rituximab and splenectomy, as well as focusing on a new approach with thrombopoietin receptor agonists (TPO-RAs). Eltrombopag and romiplostim are safe and useful drugs for paediatric ITP. These two TPO-RAs might delay surgery and other treatments such as rituximab. However, the potential side effects described in adult patients should be considered. Paediatric patients with refractory ITP, undergoing new treatments, should be supervised in specialised centres.
La trombocitopenia inmune (PTI) es una entidad relativamente frecuente en pediatría. Aunque su evolución suele ser favourable en la mayoría de los casos, el manejo de aquellos pacientes en los que la enfermedad persiste es muy controvertido. Este artículo pretende, a través de una revisión de la literatura más reciente, responder a aspectos relacionados con el tratamiento de la PTI persistente, crónica y refractaria durante la infancia, haciendo especial énfasis en el papel del rituximab, la esplenectomía y los análogos de la trombopoyetina (ar-TPO) en la infancia. La aparición de los ar-TPO (eltrombopag y romiplostim) amplía el arsenal terapéutico de la PTI pediátrica. Además, tras haber demostrado un perfil de seguridad adecuado en ensayos clínicos, retrasa la indicación de esplenectomía o el uso de tratamientos asociados a mayor riesgo de complicaciones, como rituximab. No obstante, se recomienda que su manejo sea supervisado por centros con experiencia de cara a monitorizar complicaciones potenciales a medio y largo plazo ya descritas en el paciente adulto.
Immune thrombocytopaenia (ITP) is an acquired immune-mediated disorder characterised by an isolated thrombocytopaenia secondary to destruction of platelets mediated by autoantibodies. Unlike adults, most paediatric patients have a benign course that is self-limiting and a lower risk of bleeding. However, the disease may become chronic in up to 20% of patients. Based on the duration of the disease, ITP is classified as newly diagnosed (<3 months’ duration), persistent (3–12 months) or chronic (>12 months).1
Although ITP is relatively frequent in children current diagnosis and treatment guidelines1–3 have not succeeded in establishing the optimal treatment strategy at certain stages of disease. The most controversial aspects concern to the management of patients with persistent or chronic ITP and children who do not respond to first-line treatment (regardless of disease duration).
The development of new drugs indicated for its use in children motivated us to write this article, where we attempt to address several issues in the treatment of persistent, chronic and refractory ITP in children, and the position of these novel agents in the treatment algorithm.
How are persistent, chronic and refractory ITP defined and diagnosed in the paediatric age group?ITP is defined as persistent when thrombocytopaenia continues after 3–12 months from the initial diagnosis.2,4 However, there is less agreement in the definition of refractory ITP. In adults, refractory ITP is defined as a disease that does not respond or relapses after splenectomy, a criterion that cannot be applied to the paediatric population, since surgery is not considered a second-line treatment in many cases.5 Thus, it has been proposed that this definition includes children with significant bleeding that do not respond to first-line treatment or without significant bleeding in whom the primary objective of treatment is to improve the quality of life.
We ought to highlight that in either case, and regardless of whether the patient has ever experienced spontaneous remission, there is still a high probability that spontaneous remission will occur within a year from diagnosis.6 This is important in regard to following steps as surgical treatment (splenectomy) or treatments associated with a higher incidence of complications (such as immunodeficiency secondary to use of rituximab) should be avoided if possible.
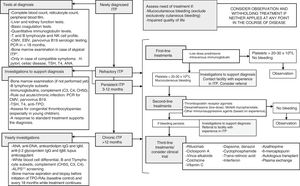
At any rate, diagnosis needs to be confirmed before developing a treatment plan5 (Fig. 1). In infants aged <1 year, the possibility of congenital thrombocytopaenia or ITP secondary to an underlying immunodeficiency must be contemplated. A history of recurrent infection or the presence of concomitant immune-mediated cytopenias should lead to suspicion of a primary immunodeficiency. Evaluation by a clinical immunologist is advisable in these cases. It is also important to take into account that ITP may be secondary to other autoimmune disorders. Thus, systemic lupus erythematosus, antiphospholipid syndrome and other autoimmune disorders of connective tissue should be ruled out in patients with compatible presentations (regardless of the duration of disease) and once a year, at minimum, in all patients with chronic ITP (especially in female adolescents or after a prolonged course of disease).1,3
Approach to the diagnosis and management of ITP in patients that are not eligible for splenectomy (or did not respond to surgical intervention). *Typical ITP: patient aged 1–10 years, with sudden onset of symptoms, afebrile, with a history of infection or vaccination in the 2 preceding weeks and with isolated thrombocytopaenia that cannot be explained by findings of the physical examination other than haemorrhagic symptoms. CMV, cytomegalovirus; EBV, Epstein–Barr virus. **ALPS, autoimmune lymphoproliferative syndrome. Only needs to be ruled out once by evaluation of CD4-CD8-double-negative T cells.
When it comes to paediatric patients with ITP, the most important step prior to initiating treatment is to assess whether it is justified by haemorrhagic manifestations. Thus, treatment should be reserved for patients with active bleeding (excluding exclusively cutaneous bleeding). On the other hand, prevention of bleeding in asymptomatic patients would only be justified in the case of surgical intervention or if the clinician in charge believes that there is a high risk of bleeding associated to the physical activity of the child. Other factors, such as the circumstances of the family (parental anxiety or socioeconomic status, among others) or the quality of life of the patient may also be taken into account in treatment planning, always balancing the risks and benefits of treatment.7
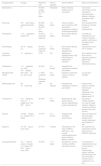
If after an appropriate assessment, treatment is indicated, administration of first-line drugs (immunoglobulins and prednisone) is the recommended initial strategy, avoiding administration of corticosteroids for longer than 7–10 days. Administration of anti-D immunoglobulin should be avoided due to the previously reported cases of severe haemolytic anaemia following its use.1 Thus, second-line treatments should only be used in patients who do not respond to first-line treatment or that require prolonged steroid therapy to achieve an adequate responses.3,4 They include immunosuppressive therapy, rituximab, thrombopoietin receptor agonists (TPO-RAs) and splenectomy. Table 1 summarises the most frequently treatments used in cases of persistent and refractory ITP with their corresponding dosages and response rates. In addition, Fig. 1 presents the three tiers of medical treatment recommended for ITP.5Immunosuppressive therapy with drugs such as ciclosporin, dapsone, dexamethasone or mycophenolate is well tolerated and does not seem to produce significant complications. So far, there is no evidence of a higher response rate with one of them compared to others.5 Furthermore, while the experience in real-world clinical practice may support their use, these drugs have not been authorised by regulatory agencies for treatment of ITP due to the available scientific evidence comes from observational and, in most instances retrospective studies. On the other hand, administration of chemotherapeutic agents such as cyclophosphamide, mercaptopurine or vincristine is increasingly discouraged, although there are references to their use in older case series.
Main drugs and procedures used in the management of persistent and refractory ITP.
| Drug/procedure | Dosage | Response rate | Time to response | Adverse effects | Special considerations |
|---|---|---|---|---|---|
| Splenectomy | – | 70–85% initially, 60–75% at 5 years | Immediate | Risk of infection. Potential risk of thrombosis | Vaccination according to current schedule for splenectomised patients. Antibiotic prophylaxis after surgery. |
| Rituximab | 375mg/m2/dose, weekly doses (x4), IV | 60–68% initially, 20–25% at 5 years | 1–8 weeks | Infusion reaction, serum sickness, HBV reactivation, progressive multifocal leukoencephalopathy | Not indicated for this disease in summary of product characteristics |
| Romiplostim | 1–10μg/kg/dose weekly, SC | 40–50% sustained response | 1–4 weeks | Bone marrow fibrosis, thrombosis | Currently not indicated for use in children. Requires weekly monitoring at the beginning of treatment |
| Eltrombopag | 25–75mg/day p.o. | 40–50% sustained response | 1–2 weeks | Bone marrow fibrosis, thrombosis, hepatotoxicity | Indicated for chronic ITP in children |
| Low-dose prednisone | <5mg/day p.o. | <10% | N/A* | Weight gain, hyperglycaemia, high blood pressure, osteoporosis, cataracts | Not initiated at a low dose: dose is gradually lowered in patients that respond to intermediate doses |
| Azathioprine | 1–2mg/kg/day p.o. (max 150mg/day) | 40–60% | 3–6 months | Hepatotoxicity, neutropenia, infection, pancreatitis | |
| Mycophenolate mofetil | 250–1000mg p.o. twice a day | 11–80% variability in sustained response | 4–6 weeks | Headache, diarrhoea, nausea, anorexia, infection | Usually well tolerated |
| 6-Mercaptopurine | 50–75mg/m2/day | 83% | Not reported | Hepatotoxicity, neutropenia, infection, pancreatitis | Evidence from studies in children with immune-mediated cytopenias. Few responses in patients with ITP |
| Ciclosporin A | 5–6mg/kg/day every 12h (target level, 100–200ng/mL) | 30–60% | 3–4 weeks | Nephrotoxicity, high blood pressure, tremors, paraesthesia, gingival enlargement | One study in children reported one case of fungal infection. Requires monitoring of blood pressure and renal function |
| Danazol | 50–800mg/day p.o. divided in 2–4 doses | 10–70% | 3–6 months | Hepatotoxicity, virilisation, amenorrhoea | Improved responses with prolonged treatment and durable responses with treatment >1 year |
| Dapsone | 75–100mg p.o. once a day | 40–75% | 3 weeks | Haemolysis (in the case of G6PDh deficiency), rash, nausea, methemoglobinemia, agranulocytosis, aplastic anaemia | No evidence from randomised clinical trials. Rule out G6GPDh deficiency |
| Cyclophosphamide | 0.3–1g/m2 IV every 2–4 weeks (1–3 doses), 50–200mg p.o. once a day | 24–85% | 1–16 weeks | Neutropenia, nausea, vomiting, infertility, secondary tumours | It is important to emphasise the need of fluid intake to prevent haemorrhagic cystitis. Very few published data. Not recommended if clinician has no experience in its use |
G6PDh, glucose-6-phosphate dehydrogenase; HBV, hepatitis B virus; IV, intravenous; p.o., oral route; SC, subcutaneous.5–7
There is also no evidence that rituximab is more effective than other treatments. In fact, it appeared to be less effective in some case series. Furthermore, while it has proved useful in adults and the literature describes cases with a durable response, it must be taken into account that its use can lead to serious adverse effects in children.7Thrombopoietin receptor agonists (eltrombopag and romiplostim) have also been used in patients with persistent or refractory ITP. Data on its use in paediatrics is still scarce, but both romiplostim and eltrombopag are useful in the treatment of persistent ITP, with adequate tolerability and few adverse events.8,9 Long-term response rate is similar to those reported for ciclosporin, dapsone or mycophenolate, approximately 45%, but in the case of TPO-RAs data come from randomised clinical trials.10–13 In this regard, it is important to note that although the approved indication of TPO-ARs for treatment of ITP in children is limited to the chronic form, the definition of chronic ITP at the time the trials were performed included patients in whom ITP has persist more than 6 months. Thus, strictly speaking, their efficacy for treatment of persistent ITP has already been proved. In addition, it has been reported that patients that initially responded to TPO-RAs and subsequently needed to discontinue treatment did not experience a rebound of thrombocytopaenia.8 There are two possible reasons for this: these drugs can modify the course of the disease, or TPO-ARs serve as a temporising measure until the disease resolves. Regardless of which is true, the final outcome is beneficial to the patient and tips the scales in favour of the use of TPO-RAs over other treatment options. However, there are still many aspects that need to be explored, such as the use of TPO-RAs in combination therapy (low-dose steroids, immunoglobulins or even mycophenolate), or whether a clinical improvement may be considered to continue treatment regardless of the platelet count. Nevertheless, the potential adverse effects of these drugs in the medium- and long-term (addressed later in the article) and the high cost of this therapy call for its rational use, which should preferably be restricted to facilities offering paediatric specialty services.
Splenectomy continues to be the treatment with the highest response rate. The safety of this surgical procedure has improved in recent years with the use of antibiotic prophylaxis and vaccines, and improvements in the detection of the thromboembolic events that these patients may experience in the medium and long term.7 However, now that TPO-RAs, which have a better safety profile, have become available, it seems appropriate to use them before splenectomy with the aim of deferring surgery.
Is rituximab a second-line treatment option in the paediatric population?The indication of rituximab for treatment of ITP is based on its ability to control the humoral immune response through the depletion of CD20+ B cells. There is also evidence that suggests that it can modulate cell-mediated immunity by increasing the number of circulating the regulatory T cells and preventing the activity of autoreactive T cells.5
Although the summary of product characteristics of rituximab does not include the indication for treatment of ITP in children, there are many references about its use in the medical literature. One prospective study assessed the use of rituximab in 36 paediatric patients and concluded that it could be beneficial in some patients with chronic ITP.14 The predictors of a good response to rituximab include initial complete response, prolonged B cell depletion,15 good response to corticosteroids and secondary ITP.16 Another retrospective study described long term treatment outcomes (5 years) after rituximab administration in children and adults with ITP while the initial response rate was 57%, the proportion of patients with sustained responses was much lower, approximately 26%.15 This loss of response could be due to the persistence of autoreactive clones in the germinal centres and bone marrow.7
In adult patients, the current level of evidence does not justify recommending the use of rituximab in place of splenectomy as a second-line treatment. In fact, while one meta-analysis concluded that rituximab should be used before splenectomy,17 several studies have shown similar responses before and after surgery.18 Consequently, its use as a second-line treatment can only be recommended in patients in whom surgery carries a high risk or that refuse splenectomy.
The use of rituximab as a second-line treatment is even more controversial in paediatric patients. Before TPO-RAs, several publications already recommended splenectomy in patients with refractory ITP (albeit deferring it, where possible, until 12 months from onset).3,19 Furthermore, although the use of rituximab was proposed as an alternative to splenectomy in children with chronic or refractory ITP, authors emphasised its potential adverse effects—serum sickness, increased risk of viral infection, hepatitis B virus reactivation or secondary hypogammaglobulinemia—as well as other peculiarities surrounding its administration, such as the need to put vaccinations on hold for 6 months after completion of treatment.
The most recent guidelines,4,5,7,20 developed after the introduction of TPO-RAs, propose an individualised approach to treatment, although due to the lack of randomised controlled trials, their recommendations are not based on a level of evidence higher than expert opinion. Some authors continue considering rituximab an option for a second-line treatment (along with TPO-RAs), but there are others that decidedly present TPO-RAs as a preferable alternative. This is mainly because despite the lack of long-term data, the current evidence suggests that TPO-RAs produce more durable responses and few side effects. In fact, even splenectomised patients who have not responded to other lines of treatment in the past may benefit from the use of TPO-RAs after failure of splenectomy.21 Lastly, unlike rituximab, eltrombopag has been authorised for the treatment of chronic ITP in paediatric patients, as noted in its summary of product characteristics.
In conclusion, based on the current evidence, rituximab should probably not be used for second-line treatment of refractory, persistent or chronic ITP in paediatric patients. However, since treatment is established on a case-by-case basis, this type of decision should be made by a clinician with specialised knowledge and experience in this field.
What is the role of splenectomy in paediatric patients after the development of TPO-RAs?Until a few years ago, splenectomy was one of the main strategies for treatment of ITP in adults and children aged more than 5 years. After the introduction of rituximab, pharmacological treatment started to displace surgery as a second-line treatment.3,19 At present, many experts recommend the use of TPO-RAs in patients that do not respond to initial treatment with corticosteroids and immunoglobulin before the use of rituximab and surgery.20 However, studies that compare both therapeutic options have yet to be conducted.
In general, and while most guidelines do not recommend splenectomy until ITP has lasted a minimum of 12 months,6 its use remains controversial. Some patients, as those aged <5 years are not eligible for surgery, in other cases parents or legal guardians refuse splenectomy. Finally, sometimes the primary goal of treatment is to improve the quality of life (cases where medical treatment would a priori be preferred over surgical treatment).
In all other patients, those who are actually candidates for splenectomy, the risks associated with this irreversible procedure. Thus, despite a good initial response (70–85% of cases) and the fact that most patients maintain a normal platelet count 5 years post-intervention (60–70% of cases), we cannot ignore the associated morbidity7,22,23: the risk of infection in splenectomised patients is as high as 11–16% even with correct vaccination and administration of antibiotic prophylaxis, the proportion of patients that develop thrombosis after surgery ranges between 1.6 and 4.3%, and the risk of severe intraoperative or postoperative haemorrhage is of 0.78%.24
Therefore, and since the benefits of splenectomy do not seem to outweigh its disadvantages in children, this intervention should only be used in selected cases: patients that cannot tolerate or do not respond to medical treatment, including corticosteroids, immunoglobulins, TPO-RAs and even immunosuppressive therapy. Splenectomy is also clearly indicated in patients with life-threatening haemorrhage, although this is an extremely rare event. Lastly, splenectomy can also be considered in patients that have a poor health-related quality of life despite receiving other treatment, or at the request of the family.7
When splenectomy is planned (always in patients aged more than 5 years), it should be performed in units with experience in this procedure by laparoscopic approach, the patient must receive all pertinent vaccines in adherence to current guidelines before the procedure, and appropriate antibiotic prophylaxis must be administered in the postoperative period.7 We need to emphasise that, unfortunately, there is no known prognostic factor that can help predict which patients will respond to surgery.
In summary, the latest recommendations call for exhausting all options of medical treatment in children with chronic ITP before resorting to splenectomy, with especial emphasis on the use of TPO-RAs (Fig. 1).
Is long-term use of TPO-RAs safe in children?As the short- and long-term safety and efficacy of TPO-RAs for treatment of ITP of more than 6 months’ duration have been demonstrated, the use of these agents has been increase during the years. However, while we know that there are adverse events associated with the use of these drugs, the available data on adverse events in children are still scarce.
The use of eltrombopag has been associated with the development of liver function abnormalities (elevation of liver enzymes), but there is evidence that this improves after discontinuation of treatment, so this complication would not constitute a problem in the long term.25
Bone marrow fibrosis has been described in adult patients treated with both types of TPO-RAs, but the actual risk of this complication in the paediatric population remains unknown. Although clinical trials of eltrombopag in the paediatric population did not include this variable, cases of this adverse event have been reported in the literature.26 As for romiplostim, clinical trials that are still in progress are evaluating the development of fibrosis and subsequent outcomes to determine whether the observed abnormalities resolve after discontinuation of treatment, as is the case in adults.27
Given the lack of conclusive data, and although there have been no reports of cases of significant fibrosis in children (highest grades reported, I–II),9,26,28 some research groups recommend performance of serial biopsies every 18 months in order to monitor potential changes in bone marrow.26 Another aspect worth considering is that periodic examination of peripheral blood smears by an expert haematologist can help rule to out the presence of features that provide indirect evidence of myelofibrosis: anisocytosis, poikilocytosis, dacrocytes, nucleated red blood cells and polychromasia.
Treatment of TPO-RAs has also been associated with thrombosis in adult patients. However, it is still unclear whether the risk of thrombosis is related to the treatment or to the disease itself.25 We know that patients with ITP exhibit a procoagulant profile secondary to the changes generated by their underlying disease29 that has been associated with a risk of arterial and venous thrombosis (relative risk, 1.5 and 1.9, respectively).25 On the other hand, we cannot disregard the potential role of other independent risk factors for thrombosis that are frequently present in adult patients, such as diabetes mellitus, hypercholesterolemia, high blood pressure, smoking, atrial fibrillation, valvular or coronary disease, splenectomy or treatment with corticoids.30 Finally, there is evidence that treatment with TPO-RAs may be an independent risk factor for the development of thromboembolic events in adult patients (relative risk, 1.8).27 However in children, the only available evidence is the report of two cases of thrombosis related to treatment with eltrombopag in a recently published case series from the United States,9 so the actual risk of this treatment in the paediatric age group remains to be determined.
Nowadays, recommendation is to maintain a high level of suspicion to avoid delays in the detection of thrombotic complications and to take into account other risk factors, such as obesity, the use of hormonal birth control methods in adolescents or the family history of thrombosis before starting treatment.
ConclusionsSince ITP is a diagnosis of exclusion, persistence of disease or lack of response to conventional treatment calls for re-evaluation of the working diagnosis. On the other hand, the approach to the management of paediatric patients with ITP at any stage calls for watchful waiting in the absence of haemorrhagic symptoms that would justify treatment.
The introduction of TPO-RAs in clinical practice has increased the therapeutic burden available in paediatric ITP, and also stablish new approaches to patients with persistent, chronic or refractory to a first-line treatment ITP, delaying the use of more aggressive treatments like rituximab or splenectomy. However, unless a demonstrated effectiveness and the evidence of a good safety profile described in randomised clinical trials, their recent development requires that their use be monitored by experts in the field with a high level of suspicion for potential complications, unless they will probably be less frequent in the paediatric age group compared to adults.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Berrueco R, Dapena JL, Sebastián E, Sastre A. Controversias en el tratamiento de la trombocitopenia inmune pediátrica. Ann Pediatr. 2018;89:189.