To compare between 2 sedoanalgesia regimes, the time from withdrawal of the medication until the patient wakes up and until extubation.
MethodologyObservational study on pediatric patients after elective surgery that needed mechanical ventilation for a period maximum to 72 h. We compared two independent groups of patients: group A: patients collected prospectively who received sedoanalgesia with propofof-remifentanil and group B: patients who received midazolam-fentanyl collected retrospectively by reviewing medical records and database of the unit. The main variables studied were: Age, weight, sex, interventions type, sedoanalgesia scales, drugs dosages, time from withdrawal of medication to awakening and extubation, and adverse effects.
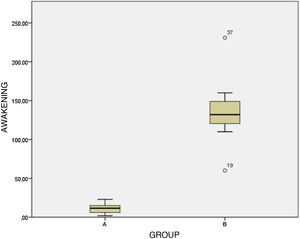
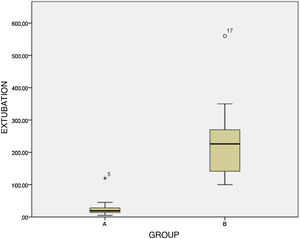
ResultsWe collected 82 patients, 43 in group A and 39 in group B. Age (arithmetical mean ± standard deviation of patients were 49 ± 65 months, weight 17 ± 16 kg. Mechanical ventilation medium time was 22 h (3–72), wake-up time from withdrawal after removing sedoanalgesia was of 11,8 ± 10,6 min group A and 137,3 ± 45 min group B (P < 0.001), extubation time after removing sedoanalgesia was of 24 ± 21 min group A and 230 ± 102 min group B (P < 0.001). Adverse effects were found in 10.5% of patients group A (7.9% agitation, 2.6% bradycardia), and 13% of patients group B (respiratrory depression after extubation) P = 0,572.
ConclusionsPatients treated with propofol-remifentanil have significantly shorter times to wake up, extubation and withdrawal from mechanical ventilation after stopping the medication. In the midazolam-fentanyl group, respiratory depression was more frequent, although the percentage of adverse effects were similar in both groups. Both the combination of propofol-remifentanil and midazolam-fentanyl appear to be effective as a sedative-analgesic regimen for patients undergoing mechanical ventilation after surgery.
Comparar, entre 2 regímenes de sedoanalgesia, el tiempo trascurrido desde la retirada de la medicación hasta el despertar y hasta la extubación del paciente.
MetodologíaEstudio observacional, en pacientes pediátricos que, tras cirugía electiva, precisaron sedoanalgesia y ventilación mecánica durante un periodo máximo de 72 h. Comparamos dos grupos independientes de pacientes: grupo A, pacientes que recibieron sedoanalgesia con propofol-remifentanilo recogidos de forma prospectiva; y grupo B, pacientes que recibieron midazolam-fentanilo recogidos retrospectivamente mediante la revisión de las historias clínicas y base de datos de la unidad.
Las variables estudiadas fueron: edad, peso, sexo, tipo de intervención, escalas de valoración de la sedoanalgesia, dosis totales empleadas, tiempo transcurrido desde la retirada de medicación hasta despertar y extubación, y efectos adversos.
ResultadosSe recogieron 82 pacientes, 43 en el grupo A y 39 en el grupo B. La edad (media ± desviación estándar) de los pacientes fue de 49 ± 65 meses y 17,3 ± 16 kg de peso, con un tiempo de ventilación mecánica promedio de 22 horas (3–72). Tras retirar la medicación, el tiempo de despertar fue de 11,8 ± 10,6 min en el grupo A y de 137,3 ± 45 min en el grupo B (p < 0,001) y el tiempo de extubación de 24 ± 21 min en el grupo A y 230 ± 102 min en el B (p < 0,001). El 10,5% de los pacientes del grupo A presentó algún efecto adverso (7,9% agitación y 2,6% bradicardia) y, del grupo B, un 13% (depresión respiratoria tras extubación), con una p = 0,572.
ConclusionesLos pacientes tratados con propofol-remifentanilo tienen un tiempo promedio significativamente menor de despertar, extubación y retirada de ventilación mecánica tras suspender la medicación. En el grupo de midazolam-fentanilo, fue más frecuente la existencia de depresión respiratoria, aunque el porcentaje de efectos adversos es similar en ambos grupos. Tanto la combinación de propofol-remifentanilo como midazolam-fentanilo parecen ser efectivas como pauta sedoanalgésica de pacientes sometidos a ventilación mecánica tras intervención quirúrgica.
One of the important goals of paediatric intensive care units (PICUs) is the adequate management of postoperative pain, especially in patients under mechanical ventilation.
Sedation and analgesia are key elements in the management of critically ill children1 and necessary during endotracheal intubation and mechanical ventilation. Children that need mechanical ventilation in the immediate postoperative period usually require analgesia to alleviate pain and sedation to maintain comfort, safety and adequate adaptation to the ventilator. The most frequently used analgesic in our region is fentanyl, and benzodiazepines, especially midazolam, are the most frequently used sedatives. Some of the other drugs commonly used are morphine hydrochloride, clonidine and ketamine.
However, classic analgesics and sedatives like fentanyl and midazolam have a cumulative effect when administered as continuous infusion, which increases their half-life and results in prolonged sedation and an unpredictable extubation time after discontinuation of infusion. Children frequently wake slowly, do not adapt to the ventilator and have breathing impulses and airway reflexes that are insufficient for extubation, hindering weaning and increasing the risk of accidental extubation during this stage.2,3 Weaning is defined as the transition from ventilatory support to full spontaneous breathing, during which the patient is responsible for gas exchange while ventilatory support is removed, and ending with the extubation process.4 Therefore, an optimal sedation and analgesia regimen should provide adequate sedation and analgesia while the child is receiving mechanical ventilation and facilitate rapid recovery and weaning after the medication is discontinued.
For this reason, we compared 2 sedation/analgesia regimens, one with frequently used drugs (midazolam/fentanyl) and one with drugs with a shorter half-life (propofol/remifentanil), with the hypothesis that the time to awakening and to extubation after discontinuation of treatment would be shorter in patients treated with propofol-remifentanil.
The primary objective of our study was to analyse the time elapsed from discontinuation of sedation/analgesia to awakening and to extubation in the 2 groups.
The secondary objective was to analyse and compare the incidence of adverse events, including the development of hyperlacticaemia, in the 2 groups of patients.
MethodsWe conducted an observational study in the PICU of a tertiary care hospital in patients that required mechanical ventilation for a maximum of 72 h after undergoing elective surgery.
We compared 2 separate groups of patients:
- –
Group A: patients enrolled prospectively that received sedation/analgesia with propofol-remifentanil between November 2014 and June 2016.
- –
Group B: patients included retrospectively through the review of health records and the PICU database that received midazolam-fentanyl between June 2012 and October 2014.
- –
Haemodynamically stable.
- –
Age 1–18 years.
- –
Mechanical ventilation required for less than 72 h in the postoperative period.
- –
Signed informed consent of parents or legal guardians.
- –
Known intolerance or allergy to any of the drugs.
- –
Patients that underwent cardiovascular surgery.
- –
Propofol: intravenous infusion starting at a rate of 1 mg/kg/h, with progressive increases of 0.5 mg/kg/h if the Ramsay sedation scale score was less than 4 until a score of 5 was achieved.
- –
Remifentanil: intravenous infusion starting at a rate of 10 μg/kg/h, with progressive increases of 5 μg/kg/h if the pain scale score was greater than 2 until adequate analgesia was achieved (pain scale score ≤ 2).
- –
Midazolam: intravenous infusion starting at a rate of 0.2 mg/kg/h, with progressive increases of 0.5 mg/kg/h if the Ramsay sedation scale score was less than 4 until the optimal score of 5 was achieved.
- –
Fentanyl: intravenous infusion starting at a rate of 2 μg/kg/h, with progressive increases of 0.5 μg/kg/h if the pain score was greater than 2.
If the patient experienced severe pain (pain scale score > 6) or agitation (Ramsay scale score of 2–3), rescue boluses of the drug applicable to the study group were delivered intravenously: remifentanil at 1 μg/kg or midazolam at 0.1 mg/kg in group A, and fentanyl at 1 μg/kg or propofol at 1 mg/kg in group B.
Assessment of analgesiaWe used the Multidimensional Assessment Pain Scale (MAPS) in patients under deep sedation, applied by a paediatrician or nurse in the unit. If the patients were awake, we used the MAPS scale in children aged 0–3 years, the Wong-Baker FACES scale in children aged 3–7 years and the Visual Analog Scale in children aged more than 7 years. Pain was assessed on a 0–10 scale (0 no pain, 1–2 mild pain, 3–6 moderate pain, 7–8 intense pain, 9–10 unbearable pain). The target of analgesia was a score of 2 or less.
Assessment of sedationWe used the Ramsay sedation scale, which has been recently validated for monitoring sedation during invasive procedures involving deep sedation and analgesia in the paediatric population.5 The scale establishes 6 levels of sedation. Level 1: awake, anxious, agitated or restless; level 2: awake, cooperative, oriented and tranquil; level 3: appears asleep, responding to commands only; level 4: appears asleep, with brief responses to light and sound; level 5: asleep, responsive only to painful stimuli; level 6: deeply asleep and unresponsive to stimuli. The objective was to maintain the level of sedation at 5.
Assessment of time of awakeningWe defined the time of awakening as the time patients reached a Ramsay scale score of less than 3 after discontinuation of medication.
Patient monitoringAll patients underwent continuous monitoring of systolic blood pressure, diastolic blood pressure, mean arterial pressure, heart rate, respiratory rate and transcutaneous oxygen saturation.
Study variables- –
Age.
- –
Weight.
- –
Sex.
- –
Type of surgery.
- –
Assessment of pain and sedation.
- –
Time elapsed from discontinuation of sedation/analgesia to awakening.
- –
Time elapsed from discontinuation of sedation/analgesia to extubation.
- –
Need of reintubation.
- –
Doses of all drugs used.
- –
Need of rescue doses of sedatives or analgesics.
- –
Adverse events (accidental extubation, reintubation, respiratory depression, need of reversal, other).
- –
Lactate level in peripheral blood at admission and at 24 h (mmol/L, normal range: 0.6−2 mmol/L).
We performed a basic descriptive analysis of the study variables. We calculated absolute and relative frequencies for qualitative variables. Depending on whether the data were normally distributed or not, which we determined using the Kolmogorov–Smirnov or the Shapiro–Wilk (n < 50) test, we summarised quantitative variables as mean ± standard deviation (SD) and range (minimum–maximum) or median and interquartile range (IQR), respectively. We compared qualitative data in the study groups by means of the χ2 or Fisher exact test, and quantitative data with the Student t test for independent samples or the Mann–Whitney U test depending on whether the data followed a normal distribution. We defined statistical significance as a p-value of less than 0.05. The statistical analysis was performed with the software PASW Statistics version 18.0 (IBM® SPSS Statistics v18.0).
Ethical considerationsPatients were assigned a numerical code, and only the researchers that conducted the study had access to personally identifiable data, maintaining the confidentiality of participants in adherence with Organic Law 15/1999.
The study was approved by the Ethics Committee of the Autonomous Community of Andalusia.
ResultsThe study included a total of 82 patients, 43 in group A and 39 in group B.
Table 1 describes the general characteristics of the patients.
General characteristics of the patients. Group A: sedoanalgesia con propofol-remifentanil; group B: sedoanalgesia con midazolam-fentanyl.
| Age | 49 ± 65 meses | ||
| Weight | 17.3 ± 16 kg | ||
| Sex | 56% female | ||
| 44% male | |||
| Type of surgery | Maxillofacial | 71 patients (86.5%) | |
| Trauma | 8 patients (9.5%) | ||
| Neurosurgery | 3 patients (4%) | ||
| Time to awakening after discontinuation | Group A | 11.8 ± 10.6 min | P < .001 |
| Group B | 137.3 ± 45 min | ||
| Time to extubation after discontinuation | Group A | 24.2 ± 21 min | P .001 |
| Group B | 230 ± 102 min | ||
| Adverse events | Group A | 10.5% of patients | P > .05 |
| Group B | 13% of patients | ||
| Lactate levels | Group A | Admission, 1.22 ± 0.3 mmol/L | P > .05 |
| Group B | 24 h, 0.89 ± 0.3 mmol/L | ||
| Admission, 1.75 ± 0.3 mmol/L | |||
| 24 h, 0.76 ± 0.3 mmol/L | |||
| Need of rescue doses | Group A | 53% (23 patients) | P > .05 |
| Group B | 51% (20 patients) | ||
| Scale sores before rescue | Ramsay scale | 1−3 (2.2 ± 0.7) | |
| Pain scale | 7−9 (7.5 ± 0.6) | ||
| Scale scores after rescue | Ramsay scale | 5−6 (5.2 ± 04) | |
| Pain scale | 0−2 (1.5 ± 0.9) | ||
| Patients that did not require rescue | Ramsay scale | 4−5 (4.5 ± 0.5) | |
| Pain scale | 0−3 (1.7 ± 1) |
Ramsay scale levels: 1–6; pain scale levels:0–10.
The time to awakening after discontinuation of sedation/analgesia (Fig. 1) was significantly shorter in group A compared to group B (11.8 ± 10.6 min vs 137.3 ± 45 min; P < .001. On the other hand, the time to extubation after discontinuation of the medication (Fig. 2) was 24.2 ± 21 min in group A compared to 230 ± 102 min in group B, a difference that was also statistically significant (P < .001).
In group A, 10.5% of the patients experienced adverse events (4 patients accidental extubation, 3 patients agitation, 2 apnoea and 1 low blood pressure), none requiring reintubation. In group B, 13% of the patients experienced adverse events. Five of them developed respiratory depression after extubation, of who 2 required reintubation and 3 reversion of depressor effects with naloxone and flumazenil. In addition, 3 patients in this group experienced accidental extubation. We did not find significant differences between groups (P = .572).
Table 2 presents the dosage of the drugs administered to the patients, expressed as the total dose (mean, range) of each drug, including rescue doses, required to achieve the established analgesia and sedition targets (pain scale score < 3, Ramsay sedation level 5).
The serum lactate levels measured at admission and at 24 h were in the normal range in every patient in the total sample, without significant differences between groups (P = .93) (Table 1).
DiscussionWhen we analysed the outcomes under study, we found that the times to awakening and to extubation after discontinuation of sedation/analgesia were significantly shorter in the propofol-remifentanil group compared to the midazolam-fentanyl group, with results similar to those reported by Welzing et al.6.
The most widely used combination of drugs is fentanyl or morphine for analgesia and midazolam for sedation,7 which achieve adequate levels of sedation and analgesia in paediatric patients that require mechanical ventilation after surgery.8 However, these drugs have a longer half-life, which may complicate extubation after their discontinuation and result in the need to reintubate or administer specific antidotes for reversion, as was the case in some of our patients. This is the reason that clinicians sometimes choose to give analgesics and sedatives with shorter half-lives, such as remifentanil and propofol, which can shorten the time to awakening and extubation after the medication is discontinued.
Based on the study conducted by Welzing et al.,6 neonates and infants aged less than 60 days metabolise commonly used opiates like fentanyl and morphine slower, which, added to the accumulation of these drugs due to the immaturity of the liver, carries a risk of respiratory depression upon discontinuation of the opiate. Remifentanil could be very useful in these patients, as it does not accumulate when administered as continuous infusion and is metabolised by nonspecific esterases that exhibit a high metabolic activity even in preterm infants,9,10 which explain the shorter time to awakening and extubation,7 including in adults.11 However, remifentanil has not proven superior to fentanyl in achieving and maintaining adequate analgesia.12
In agreement with the study by Welzing et al.,13 we found few adverse events, with a similar incidence in both groups (10.5% in group A and 13% in group B). Still, we ought to mention that adverse events were more severe in the fentanyl-midazolam group, as several patients experienced respiratory depression post extubation, requiring reversion with naloxone and flumazenil, and even reintubation in 2 cases.
Another sedation and analgesia strategy that needs to be considered is the one employed in the study by Welzing et al.,13 who switched from fentanyl-midazolam to remifentanil-propofol at the end of the weaning phase, thereby achieving a rapid transition from hypnosis to an appropriate level of alertness and regular spontaneous breathing.
Propofol and remifentanil are widely used both in children and adults for anaesthesia induction and maintenance and for sedation in short operations or procedures.14–17 In association with dexmedetomidine, they can be used to reduce the dose of opioids and benzodiazepines.18 Combining ketamine with remifentanil or propofol is also effective and safe, achieving adequate sedation and analgesia in procedures such as colonoscopy, although the scores were significantly better in the remifentanil-ketamine group compared to the propofol-ketamine group.19 In mechanically ventilated children, the combination of remifentanil and midazolam achieves rapid sedation and analgesia, improves the effect of mechanical ventilation and allows a reduction in the dose of sedatives compared to midazolam as monotherapy, in addition to being well tolerated.7
Despite the effectiveness of propofol, its use in continuous infusion, especially in children, has been limited due to the risk of the so-called propofol infusion syndrome, on account of which patients need to be closely monitored in order to suspend administration of propofol if abnormalities such as metabolic acidosis or hyperlactatemia of unknown aetiology develop.20 This is the reason that lactate levels were monitored in our patients, although hyperlactataemia was not detected in any in either group. This was consistent with the findings of previous studies, including some in which propofol was used for long periods of time without evidence of development of tolerance or adverse events. The risk is highest when continuous infusion lasts longer than 48 h and the dose is greater than 4 or 5 mg/kg/h, although the syndrome has been described in patients receiving lower doses or even a single dose.1
Although the dosage of propofol, fentanyl and midazolam is well established, this is not the case of remifentanil, for which very different doses ranging from 6 to 120 μg/kg/h have been recommended,21 although they do not usually exceed 30 μg/kg/h.6,22 In our study, the total doses required to achieve adequate sedation/analgesia were as follows: remifentanil 28 ± 20 μg/kg/h, propofol 3 ± 2 mg/kg/h, fentanyl 3 ± 0.9 μg/kg/h and midazolam 0.5 ± 1.3 mg/kg/h.
The chief limitations of this study are the retrospective inclusion of some of the patients and the relatively small sample size, due to which it is difficult to prove the superior effectiveness of one or the other sedation/analgesia regimens. Although few patients received neuromuscular blocking agents, we were not able to determine who these patients were, specifically.
The main strength of the study is that the data corroborated our hypothesis, leading to the conclusion that the time elapsed to awakening, extubation and withdrawal of mechanical ventilation after discontinuation of medication was better in patients treated with propofol-remifentanil compared to those given midazolam-fentanyl.
The frequency of adverse events was low and similar in both groups, although clinically significant respiratory depression was more frequent in the group treated with midazolam-fentanyl, in which some patients required specific antidotes for reversion of drug effects and even reintubation.
While both propofol-remifentanil and midazolam-fentanyl seem to be effective combinations for sedation and analgesia in patients requiring short-term postoperative mechanical ventilation, randomised studies in larger samples are required to confirm this conclusion.
Data availabilityOur open-access data is available via Zenodo (https://doi.org/10.5281/zenodo.5794358) but we were unable to link this data set.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: López Castilla JD, Sánchez Fernández N, Charlo Molina MT, Vázquez Florido A, Murillo Pozo MA, Sánchez Ganfornina I, et al. Sedoanalgesia con midazolam/fentanilo vs. propofol/remifentanilo en postoperatorio inmediato con ventilación mecánica de corta duración. An Pediatr (Barc). 2022;96:115–121.