Neonatal units are one of the hospital areas most exposed to committing treatment errors. A medication error (ME) is defined as the avoidable incident secondary to drug misuse that causes or may cause harm to the patient.
The aim of this paper is to present the incidence of ME (including feeding) reported in our neonatal unit and its characteristics and possible causal factors. A list of the strategies implemented for prevention is presented.
Material and methodsAn analysis was performed on the ME declared in a neonatal unit.
ResultsA total of 511 MEs were reported over a period of seven years in the neonatal unit. The incidence in the critical care unit was 32.2 per 1000 hospital days or 20 per 100 patients, of which 0.22 per 1000 days had serious repercussions. The MEs reported were, 39.5% prescribing errors, 68.1% administration errors, 0.6% were adverse drug reactions. Around two-thirds (65.4%) were produced by drugs, with 17% being intercepted. The large majority (89.4%) had no impact on the patient, but 0.6% caused permanent damage or death. Nurses reported 65.4% of MEs. The most commonly implicated causal factor was distraction (59%). Simple corrective action (alerts), and intermediate (protocols, clinical sessions and courses) and complex actions (causal analysis, monograph) were performed.
ConclusionsIt is essential to determine the current state of ME, in order to establish preventive measures and, together with teamwork and good practices, promote a climate of safety.
Las unidades neonatales son uno de los ámbitos hospitalarios más expuestos a la comisión de errores de tratamiento. El error de medicación (EM) se define como el incidente, evitable, secundario a la utilización inapropiada de medicamentos, que causa o puede causar daño al paciente.
El objetivo de este trabajo es dar a conocer la incidencia de EM (incluida la alimentación) notificados en nuestra unidad neonatal así como sus características y posibles factores causales. Así mismo se expone una relación de las estrategias llevadas a cabo para su prevención.
Material y métodosSe analizan los EM declarados en un servicio de neonatología.
ResultadosDurante un período de 7 años, en el servicio de neonatología se han notificado 511 EM. La incidencia en la unidad de críticos fue de 32,2 por 1.000 días de hospitalización o 0,2 por paciente, de los cuales 0,22 por 1.000 días tuvieron repercusión grave; el 39,5% fueron errores de prescripción, el 68,1% de administración y el 0,6% reacciones adversas a medicamentos. El 65,4% fue producido por fármacos. Se interceptó el 17%. El 89,4% no tuvo repercusión sobre el paciente; el 0,6% causó secuelas permanentes o muerte. Los profesionales de enfermería declararon el 65,4% de los EM. El factor causal más frecuentemente implicado fue la distracción (59%). Se realizaron medidas correctoras simples (alertas), intermedias (protocolos, sesiones clínicas, cursos) y complejas (análisis causales, monografía).
ConclusionesEs imprescindible conocer la propia realidad para poder establecer medidas preventivas y, junto al trabajo en equipo y las buenas prácticas, promover un clima de seguridad.
Neonatal units, and especially the sections devoted to intensive care, are among the hospital settings where treatment errors are most likely to occur. Among the latter, medication errors (MEs) are part of an emergent pathology that has received increasing attention from health professionals, organisations and authorities in recent years,1 as they constitute a serious health problem in hospitalised patients,2 and one that is underestimated in the paediatric and neonatal populations.3
A ME is defined as any preventable event secondary to the misuse of a medication that may cause or lead to patient harm4; on the other hand, an adverse drug event (ADE) is defined as any harm, severe or mild, caused by the use (or lack thereof) of a drug,5 and an adverse drug reaction as a non-preventable response to a drug which is noxious and unintended and which occurs at doses normally used in man for prophylaxis, diagnosis, or therapy of disease or for modification of physiological function.6,7
It is estimated that MEs occur eight times more often in neonatal intensive care units (NICUs) than in adults in hospital.8 The drug administration protocols in the neonatal period diverge from those applied in other age groups due to the lack of dosage forms fitting the needs of neonates,8,9 their different physiology, and the fact that they are a population of heterogeneous and changing individuals that require different regimens depending on their pathology, weight, gestational age and days of life. Treating these patients requires measuring small volumes, dividing unit doses into fractions and performing complex dilutions, and the bioavailability after such manipulations is often unknown and unpredictable and may result in the use of toxic or ineffective doses. This, compounded by the variability in the pharmacokinetic and pharmacodynamic processes of the drugs (preservatives, stabilisers, etc.),9 the greater severity of disease, the longer length of stay and the impossibility of communicating with the patient among other factors make detecting errors more difficult. In this regard, Cotrina Luque et al. have developed a model list of high-risk drugs in the neonatal and paediatric populations, which they defined as those that may cause severe harm or even death when misused.10
Medication errors can occur at different points in care delivery, from the time of prescribing to the time of administration. Although prescribing, dispensing and administration are particularly important in the neonate, the causes of errors11 often occur earlier (labelling, packaging, names). For all the above reasons, the frequency with which they occur12,13 and their potential seriousness, the prevention of MEs must be addressed with a multidisciplinary approach.14
It is widely accepted that improving patient safety requires identifying and analysing errors and implementing the measures needed to prevent their reoccurrence. The aim of our study was to analyse the MEs that occurred in a department of neonatology, the medications involved, the severity of the errors and the potential for preventing them, and to describe the strategies implemented for their prevention.
Materials and methodsIn the Department of Neonatology, the Committee for Patient Safety (Comisión para la Seguridad del paciente neonatal [CSPN]), which started to operate in 2008, created a voluntary AE reporting system.15 As data were collected on the reported AEs, they were analysed and preventive measures implemented. To facilitate their study, the AEs were entered into a Microsoft Office Access 2003 database and classified into events concerning patient identification, diagnosis, treatment (due to medications or other therapies), procedures (diagnostic and therapeutic) or equipment and devices.
Using the tools of the software application, we selected the MEs based on the definition given in the introduction and classified them as prescribing or administration errors; by the involved product (drugs, nutrition products, parenteral nutrition products, intravenous fluids, blood products, nitric oxide, oxygen, sucrose, others); the type of error (incorrect dose, delay in administration, incorrect route or rate of administration, medication or dose omission, inappropriate, deteriorated or expired medication, etc.); the setting where the error took place (intensive or intermediate care unit, etc.). Due to the importance of nutrition for the adequate somatic and neurologic development of the preterm neonate, nutrition products are considered as medication in this early stage of life.13,16 With regard to the health care staff, we collected data on the type of professional that made the report, whether the reporter was directly involved or not in the AE, and the reporter's seniority (<5 years, 5–10 years, >10 years). We analysed the contributing factors related to the health professionals (distraction, repetitiveness, inadequate training, personal factors, communication problems), organisational factors (excessive workloads, lack of protocols, infrastructure, environmental factors, human resources, other causes) and factors related to equipment and devices. We assessed the impact on the patient according to the scale developed by Hartwig et al.17 We classified the measures that were adopted to correct these errors into three categories: simple (direct notification, safety alerts, correct labelling, use of posters, checking equipment and materials, etc.), intermediate (protocols, monthly bulletin, clinical sessions, courses, etc.) and high-level (manuals, root cause analyses, annual conferences, prospective studies, round tables, etc.).
In the analysis of MEs, we studied whether they had been intercepted, that is, whether they had been detected before reaching patients, and whether they were preventable. We also analysed preventability, retrospectively applying an adaptation to neonatology of the table proposed by Otero-López et al.,2 which in turn was an adaptation of the questionnaire developed by Schumock and Thornton.18
ResultsWe analysed the AEs reported between February 1, 2008 and January 31, 2015. We classified the 4575 newborns admitted to the neonatology department for a total of 73,840 patient days during this seven-year period based on whether they stayed in the intensive care unit (2212 patients admitted for 13,579 patient days) or the intermediate care unit (4575 patients admitted for 60,261 days). Of the 1628 reported AEs, 511 (31.4%) were MEs (6 MEs per month under study). The incidence of MEs reported in the intensive care unit was 32.2 per 1000 patient days (19.7 MEs per 100 admitted newborns). Of all the MEs we identified, 39.5% were prescribing errors and 68.1% administration errors, while 3 events (0.6%) were finally classified as ADRs (unpreventable) as opposed to MEs.
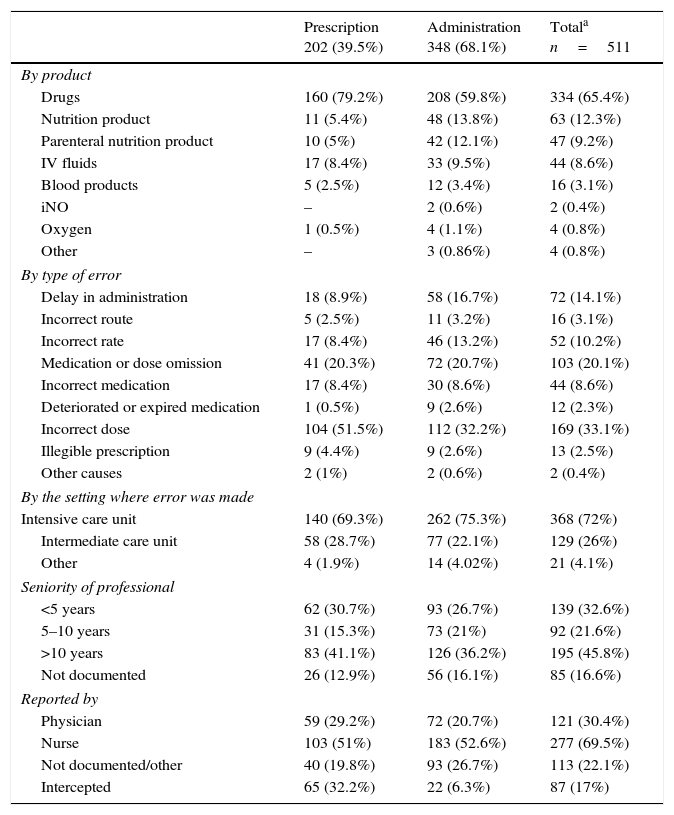
Table 1 presents the characteristics of the errors: product involved, type of error, setting of error, seniority of involved professional, type of professional that made the report, and whether the error was intercepted. It is worth noting that 65.4% of MEs involved drugs, most frequently antibiotics (86 MEs), and gentamicin (29 MEs) and vancomycin (17 MEs) in particular, with most errors consisting of wrong dosing frequency. Thirty-four reports involved sedation and analgesia (the product involved in 26 was fentanyl). Other involved drugs were caffeine (22 MEs), diuretics (18 MEs), eye drops (14 MEs), inotropes (12 MEs), insulin (12 MEs) and indometacin (6 MEs).
Characteristics of reported medication errors.
| Prescription 202 (39.5%) | Administration 348 (68.1%) | Totala n=511 | |
|---|---|---|---|
| By product | |||
| Drugs | 160 (79.2%) | 208 (59.8%) | 334 (65.4%) |
| Nutrition product | 11 (5.4%) | 48 (13.8%) | 63 (12.3%) |
| Parenteral nutrition product | 10 (5%) | 42 (12.1%) | 47 (9.2%) |
| IV fluids | 17 (8.4%) | 33 (9.5%) | 44 (8.6%) |
| Blood products | 5 (2.5%) | 12 (3.4%) | 16 (3.1%) |
| iNO | – | 2 (0.6%) | 2 (0.4%) |
| Oxygen | 1 (0.5%) | 4 (1.1%) | 4 (0.8%) |
| Other | – | 3 (0.86%) | 4 (0.8%) |
| By type of error | |||
| Delay in administration | 18 (8.9%) | 58 (16.7%) | 72 (14.1%) |
| Incorrect route | 5 (2.5%) | 11 (3.2%) | 16 (3.1%) |
| Incorrect rate | 17 (8.4%) | 46 (13.2%) | 52 (10.2%) |
| Medication or dose omission | 41 (20.3%) | 72 (20.7%) | 103 (20.1%) |
| Incorrect medication | 17 (8.4%) | 30 (8.6%) | 44 (8.6%) |
| Deteriorated or expired medication | 1 (0.5%) | 9 (2.6%) | 12 (2.3%) |
| Incorrect dose | 104 (51.5%) | 112 (32.2%) | 169 (33.1%) |
| Illegible prescription | 9 (4.4%) | 9 (2.6%) | 13 (2.5%) |
| Other causes | 2 (1%) | 2 (0.6%) | 2 (0.4%) |
| By the setting where error was made | |||
| Intensive care unit | 140 (69.3%) | 262 (75.3%) | 368 (72%) |
| Intermediate care unit | 58 (28.7%) | 77 (22.1%) | 129 (26%) |
| Other | 4 (1.9%) | 14 (4.02%) | 21 (4.1%) |
| Seniority of professional | |||
| <5 years | 62 (30.7%) | 93 (26.7%) | 139 (32.6%) |
| 5–10 years | 31 (15.3%) | 73 (21%) | 92 (21.6%) |
| >10 years | 83 (41.1%) | 126 (36.2%) | 195 (45.8%) |
| Not documented | 26 (12.9%) | 56 (16.1%) | 85 (16.6%) |
| Reported by | |||
| Physician | 59 (29.2%) | 72 (20.7%) | 121 (30.4%) |
| Nurse | 103 (51%) | 183 (52.6%) | 277 (69.5%) |
| Not documented/other | 40 (19.8%) | 93 (26.7%) | 113 (22.1%) |
| Intercepted | 65 (32.2%) | 22 (6.3%) | 87 (17%) |
When it came to the type of error, the most frequent was incorrect dosing (33.1%). The errors were intercepted in 17% of cases. Most MEs occurred in the intensive care unit (72%). The group of professionals for which errors were least frequently reported was the group with 5–10 years of seniority (21.6%). Nursing staff made 69.5% of the reports.
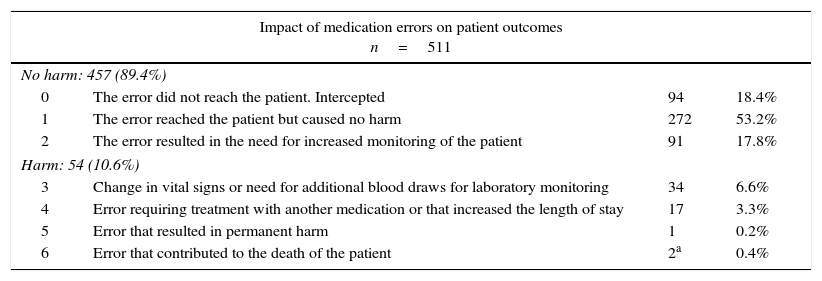
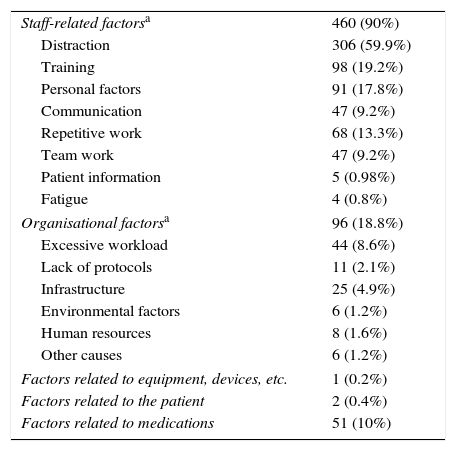
Table 2 summarises the impact on the patient according to the severity-index scale developed by Hartwig et al.17 Of all errors, 10.6% harmed the patient (8.32 MEs per 1000 patient days), and 0.6% (0.22 per 1000 days) may have contributed to the development of permanent sequelae or death (severity levels 5–6). The possible causes for MEs are summarised in Table 3. Factors related to the health care professional were documented in 89% of the reports, with distraction being the most frequent cause (59%). Of the errors related to organisational factors (18.8%), most were due to excessive workloads (8.6%).
Impact of medication errors on patient outcomes based on the Hartwig scale.
| Impact of medication errors on patient outcomes n=511 | |||
|---|---|---|---|
| No harm: 457 (89.4%) | |||
| 0 | The error did not reach the patient. Intercepted | 94 | 18.4% |
| 1 | The error reached the patient but caused no harm | 272 | 53.2% |
| 2 | The error resulted in the need for increased monitoring of the patient | 91 | 17.8% |
| Harm: 54 (10.6%) | |||
| 3 | Change in vital signs or need for additional blood draws for laboratory monitoring | 34 | 6.6% |
| 4 | Error requiring treatment with another medication or that increased the length of stay | 17 | 3.3% |
| 5 | Error that resulted in permanent harm | 1 | 0.2% |
| 6 | Error that contributed to the death of the patient | 2a | 0.4% |
Source: Hartwig et al.17
Causes associated with the medication errors (n=511).
| Staff-related factorsa | 460 (90%) |
| Distraction | 306 (59.9%) |
| Training | 98 (19.2%) |
| Personal factors | 91 (17.8%) |
| Communication | 47 (9.2%) |
| Repetitive work | 68 (13.3%) |
| Team work | 47 (9.2%) |
| Patient information | 5 (0.98%) |
| Fatigue | 4 (0.8%) |
| Organisational factorsa | 96 (18.8%) |
| Excessive workload | 44 (8.6%) |
| Lack of protocols | 11 (2.1%) |
| Infrastructure | 25 (4.9%) |
| Environmental factors | 6 (1.2%) |
| Human resources | 8 (1.6%) |
| Other causes | 6 (1.2%) |
| Factors related to equipment, devices, etc. | 1 (0.2%) |
| Factors related to the patient | 2 (0.4%) |
| Factors related to medications | 51 (10%) |
We considered 99.4% of MEs preventable after reviewing them using the table adapted from Otero-López et al.2Table 4 presents the results of this analysis. The only events that could not be classified in this table were the three identified as ADRs.
Criteria used to classify medication-related adverse events (MRAEs) according to their preventability.
| A MRAE is considered potentially preventable when one or more of the following questions can be answered in the affirmative: | Total number of affirmative answers |
|---|---|
| Was the drug involved in the MRAE not considered appropriate for the patient's clinical condition? | 53 |
| Was the dose, route, and frequency of administration not appropriate for the patient's age, weight or underlying disease? | 268 |
| Was the duration of treatment longer or shorter than the one established for the indication for which the drug was prescribed? | 132 |
| Were the clinical tests required for monitoring treatment not performed? | 1 |
| Did the patient have a previous history of allergic reaction or a similar adverse reaction to the same medication or to a medication with which it is cross-reactive or that have the same mechanism of action? | 0 |
| Did the MRAE occur as a result from some type of interaction? | 1 |
| Were potentially toxic serum concentrations of the drug or other abnormal results detected in the laboratory tests performed for therapeutic drug monitoring? | 0 |
| Was the preventive treatment required to prevent the adverse reaction not performed when the patient met the criteria to receive it? | 2 |
| Did the MRAE result from poor compliance with the prescription? | 5 |
| Did the MRAE result from an error in the administration of the drug? | 1 |
| Did the MRAE result from a circumstance that could be considered an error? | 48 |
Adapted from Schumock and Thornton.1,18
The strategies implemented for the prevention of MEs included 205 simple measures (alerts, recommendations) and 299 intermediate measures (protocols, clinical sessions, courses, etc.), and the high-level strategies included four root cause analyses and the production of a monograph on the administration of intravenous drugs in the newborn, written by a group of nurses in the unit and made available in laminated format for consultation in the department and in pocket format for its distribution to every staff member. Due to the inherent characteristics of the ME identification system, it is not possible to assess the impact of these actions on the reduction of MEs.
DiscussionComparisons between studies are difficult due to the differences in the data collection methods and definitions used in each of them. According to Suresh, the number of AEs that are detected and documented depends on the strategies implemented for their identification, and considering the variability in the approaches of different studies,19 comparisons with other studies would be less pressing than finding the errors that occur in house and for prevention strategies to be accepted.
In the analysis by Snijders et al., of 4846 voluntary incident reports corresponding to 3859 admissions, 27% were medication errors,12 a figure similar to the one found in our study (32.2%). The rate of MEs is much higher in studies in which voluntary reporting is used compared to studies that use data from mandatory reporting systems (13–14.7 versus 0.97 per 1000 patients/day in the NICU).20 This could be explained by the fact that mandatory reporting focuses on errors that produce permanent harm or death while voluntary reporting includes near-misses and low-severity events, and when trigger tools are used to investigate the circumstances that may cause AEs, the number of reports is much higher than that obtained through mandatory reporting.21
Suresh et al. found that MEs (including errors involving nutrition and blood products) are the most frequent cause of AEs (47%).13 Extremely preterm newborns require optimal nutritional support for adequate development, be it fortified human milk or special formulas, so nutrition products are considered medication16 and errors involving them MEs. A recent study by De Franco et al. described a rate of errors of 43 per 100 patients in the NICU, 48% of which were administration errors.22 Ligi et al. reported an incidence of 4.9 MEs per 100 admissions. The most frequent errors were administration errors, almost all of which were programming mistakes of pumps.23 Campino et al. analysed 91 medication samples, 52 of vancomycin and 39 of tobramycin, and detected calculation mistakes in 4.6% of the samples and precision errors in 37.9%. The authors concluded that while the detected errors had not resulted in evident harm to the patient, they had the potential to cause severe complications, and underscored the urgency of improving the methods used to prepare intravenous medications at the bedside.24
In our study, administration errors (68.1%) were also more frequent than prescribing errors (39.5%), and incorrect dosing was the most frequent error in both categories (51.5%), a finding consistent with the results of a study conducted in Hong Kong paediatric wards by Rashed et al. (42.7%).25 Horri et al. found a rate of dosing errors of 3.8 and 3.1 per 100 manual prescriptions for preterm newborns less than 33 weeks’ gestational age in two NICUs, and concluded that manual prescriptions should be forbidden in neonatal units.26 In our study, 32.2% of prescribing errors were intercepted, a low percentage compared to the one found in the study by De Franco et al., in which 63% were intercepted.22
Seventy-two percent of the reported MEs took place in the NICU, which was expected on account of the polymedication of patients in this unit.
Nurses reported errors twice as frequently as physicians (69.5% compared to 30.4%). Guerrero-Aznar et al. found that nurses reported errors 11 times as frequently as physicians.27
Neither the seniority or the inexperience of health care professionals were protective factors associated with fewer errors; on the contrary, the group reported to make the least errors corresponded to professionals with 5–10 years of seniority (21.6%). In an analysis of 6749 MEs, Stavroudis et al. identified human factors as the most frequent cause of errors (68.4%),28 while in our study, we found that factors related to health care professionals were noted in 90% of the reports, and organisational factors in 18.8% of reports.
Fortunately, a high percentage of MEs did not lead to harm (89.4%), but we must succeed in reducing these too, as failing to do so is indicative of low-quality care.1 There is evidence that AEs result from a combination of deficiencies in the system and errors made by professionals.29 Analysing potential MEs is useful because it allows the identification of break points in the system as well as points where the system works, which can help intercept errors. It is widely accepted that 1%–5% of MEs may cause harm to the patient,30 so barriers must be set up to prevent their occurrence.
Multiple measures have been undertaken to reduce MEs, the most salient being the production of a monograph on the administration of intravenous drugs in newborns written by a group of nurses that work in the unit that were not members of the CSPN but were pursuing the same goals. Comparing the present and past outcomes of our institution is difficult, because as the safety culture grows, the number of voluntary reports increases, masking the progressive improvement.
The currently used strategies of the “six rights” (right individual, right medication, right dose, right time, right route, right documentation) and the “four I's” (I prepare, I administer, I document, I am responsible) are considered insufficient to succeed in reducing the incidence of MEs, as this not only requires safe medications and procedures, but also the creation of fail-safe systems with integrated safety measures and devices that can reduce errors to the maximum or ensure that they do not reach the patient.1,19
The strategies to prevent MEs must include the development and use of protocols, staff training, and the involvement of the health authorities, medicine regulatory agencies, and pharmaceutical companies.1 Drug formulations adapted to neonates, the preparation of unit doses, the specificity of routes of administration, the use of automated systems and digital identification, electronic prescription, a broad training programme,31,32 continuous monitoring of AEs32 and having a hospital pharmacist on staff31,33 contribute effectively to reducing MEs in the paediatric population and especially in the neonatal subset.34
The high technological complexity of neonatal care requires up-to-date knowledge and the protocolisation, assessment and analysis of the work performed. The detection of errors must not lead to assigning blame, but to their analysis and correction, improving communication between professionals and promoting better practices. We need not only create a safety culture, which refers to the values it is founded on, but also a safety climate, focused on the concepts of productiveness and efficacy, and with particular emphasis on the prevention of MEs.19,35
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Esqué Ruiz MT, Moretones Suñol MG, Rodríguez Miguélez JM, Sánchez Ortiz E, Izco Urroz M, de Lamo Camino M, et al. Los errores de tratamiento en una unidad neonatal, uno de los principales acontecimientos adversos. An Pediatr (Barc). 2016;84:211–217.