Respiratory distress syndrome (RDS) is the most frequent cause of respiratory distress in preterm neonates. In the management of RDS, surfactant plays a pivotal role, but there are no evidence-based recommendations for moderate/late preterm neonates (32–36 weeks).
MethodsA scientific committee developed a questionnaire with 53 questions addressing diagnosis, treatment, potential complications and future trends in RDS specifically focused on moderate and late preterm neonates. This was followed by the performance of a Delphi survey of expert neonatologists.
ResultsConsensus was reached on 98 of the 109 items. The recommendations for the diagnosis of RDS included performing a lung ultrasound and including mild respiratory distress, transient tachypnoea of the newborn, congenital pneumonia and primary pulmonary hypertension in the differential diagnosis. Most panellists agreed on the need for studies that determine the benefit/harm balance, clinical profile and methods of surfactant administration in moderate/late preterm neonates. All respondents would use the MIST approach with devices specifically designed for surfactant administration. Regarding sedation measures during MIST, most participants agreed on the use of nonpharmacological interventions and, if these proved ineffective, an opioid. All respondents agreed that moderate/late preterm neonates are at increased risk of neonatal morbidity and mortality, particularly respiratory problems, and considered the need for more specialised monitoring in hospital follow-up visits in neonates with associated risk factors or a history of complications in the neonatal period. Finally, all respondents agreed that there is a lack of studies identifying risk factors and medium-term adverse outcomes in moderate/late preterm neonates.
ConclusionThis expert consensus will help with the diagnosis and management of RDS and guide decision-making about surfactant administration in moderate/late preterm neonates.
El síndrome de distrés respiratorio (SDR) es la causa más frecuente de distrés respiratorio en los recién nacidos prematuros. El surfactante desempeña un papel fundamental en el tratamiento del SDR, pero no existen recomendaciones basadas en la evidencia en neonatos prematuros moderados/tardíos (32-36 semanas).
MétodosUn Comité Científico diseñó un cuestionario con 53 preguntas que abordaban el diagnóstico, el tratamiento, las posibles complicaciones y las tendencias futuras del SDR, específicamente en los recién nacidos prematuros moderados y tardíos. Posteriormente, se llevó a cabo una encuesta Delphi entre neonatólogos con experiencia en el campo.
ResultadosSe alcanzó consenso en 98 de los 109 ítems incluidos. Se recomendó la realización de una ecografía pulmonar y la consideración de la dificultad respiratoria leve, la taquipnea transitoria del recién nacido, la neumonía congénita y la hipertensión pulmonar primaria durante el diagnóstico diferencial. La mayoría de los panelistas coincidieron en la necesidad de realizar estudios para determinar el riesgo/beneficio, el perfil clínico y los métodos de administración de surfactante en neonatos prematuros moderados/tardíos. Se recomendó la técnica MIST con dispositivos específicamente diseñados para la administración de surfactante. La mayoría de los participantes coincidieron en recomendar el uso de procedimientos no farmacológicos de sedación durante el MIST y, en caso de ineficacia, un opiáceo. Todos los encuestados coincidieron en que los prematuros moderados/tardíos presentan mayor riesgo de morbimortalidad neonatal, en particular de problemas respiratorios, y consideraron necesario más monitorización especializada en el seguimiento hospitalario de neonatos con mayor riesgo y/o complicaciones. Por último, todos los encuestados coincidieron en la falta de estudios para identificar factores de riesgo y resultados adversos a medio plazo en neonatos prematuros moderados/tardíos.
ConclusionesEste consenso de expertos será de ayuda en el diagnóstico y manejo del SDR y en la decisión de administrar surfactantes en neonatos prematuros moderados/tardíos.
Every year, 10% of births worldwide are preterm (<37 weeks).1 Preterm birth complications are the leading cause of death among children aged less than 5 years.2 Among the common respiratory complications in preterm neonates, respiratory distress syndrome (RDS) is the most frequent cause of respiratory distress.3
Respiratory distress syndrome is caused by impaired or delayed surfactant synthesis, secretion, metabolization, and/or degradation in the immature lung. Its incidence is inversely proportional to gestational age, with a prevalence ranging from 60% to 80% in extremely preterm neonates (<28 weeks) and 15%–30% in moderate/late preterm neonates (32–36 weeks).4
Nearly 85% of preterm births each year occur are moderate/late preterm births (32–36 weeks of gestation).1 Although preterm survival rates have increased in high-income countries, preterm birth rates have been increasing since 2000.5 As a result, the number of late preterm neonate births is growing, especially in Western countries. Late preterm neonates are at higher risk of immediate mortality and respiratory morbidity, including RDS,6,7 and at higher risk of disorders in the long term, such as neurodevelopmental disorders, neurobehavioural disorders and educational problems.8–14
In preterm neonates, surfactant replacement therapy is essential for RDS management. In spite of this, there are no evidence-based recommendations for surfactant use in late preterm neonates.15,16 The aim of the present study was to gather expert opinions on the diagnosis, treatment, potential complications and future trends of RDS with a specific focus on moderate to late preterm neonates.
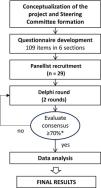
Materials and methodsStudy design and participantsThe present study involved implementation of a modified Delphi method to obtain consensus in a panel of expert specialists in neonatology.17 It was carried out in several phases, including the creation of the steering committee (SC) creation, the design of the Delphi questionnaire, the definition of the expert panel, administration of the Delphi questionnaire and data analysis and interpretation (Fig. 1).
The functions of the SC included the formulation of the questionnaire, setting the criteria for panel selection, defining the rules of consensus, interpreting the preliminary and final results and collaborating in writing the manuscript.
In regard to panel composition, highly experienced specialists in neonatology from different regions of Spain were invited to participate. The criteria for inclusion in the panel was to be a neonatologist working in a level III B/C neonatology unit in Spain with at least 10 years of experience. The list of participating panellists can be found in Supplementary Table 1.
QuestionnaireAfter defining the goals of the study, the SC carried out a literature search and developed the initial questionnaire. It included 53 questions addressing six main areas: (i) prevention/antenatal corticosteroid administration (10 items); (ii) diagnosis (9 items); (iii) treatment (72 items); (iv) sedation for minimally invasive surfactant therapy (MIST) (6 items); (v) sequelae and follow-up (7 items) and (vi) future trends (5 items). Supplementary Table 2 presents the final questionnaire. The questionnaire underwent 2 rounds of voting between February 2023 (first round) and April 2023 (second round). Participants completed the questionnaire through an online platform that ensured data anonymity and confidentiality.
Data analysis and interpretationAll items were rated on a 4-point scale: 1-totally agree, 2-basically agree, 3-basically disagree and 4-totally disagree. In the first round, consensus was defined as at least 70% of panellists selecting the same single rating category. Items for which a consensus was not reached in the first round were subject to a second round of voting. During the second round, the panellists were given the rating they had selected during the first round and the aggregated total panel results for each question. In the second round, consensus for an item was defined as at least 70% of panellists agreeing in giving a rating of 1 or 2 (consensus in agreement) or a rating of 3 or 4 (consensus in disagreement). When 60%–69% of panellists agreed in giving ratings of 1/2 or 3/4, the result was considered “undetermined-majority in agreement” or “undetermined-majority in disagreement,” and if it there was agreement below 60%, the result for the item was categorised as “no consensus.” These voting categories were used in both rounds. We conducted a statistical analysis of the responses for each question and made graphical representations of the results using Excel. The results of the Delphi survey were further evaluated and discussed by the SC.
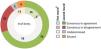
ResultsTwenty-nine highly experienced neonatologists distributed throughout Spain were included in the panel and completed the 2 rounds of the Delphi survey. At the end of the Delphi process, consensus was reached on 98 of the 109 items included in the 53 questions of the questionnaire: 76 in “agreement” and 22 in “disagreement” (Fig. 2). Of the 11 remaining items for which panellists did not reach a consensus, 6 were categorised as “no consensus,” 2 as “undetermined-majority in agreement,” and 3 as “undetermined-majority in disagreement” (Fig. 2 and Supplementary Table 2).
Prevention and antenatal corticosteroid administrationRegarding antenatal corticosteroid administration, 77% of panellists considered that the current evidence is insufficient to adequately establish the balance of benefits and harms in threatened preterm labour at 34–36 weeks of gestation, and 73% agreed not to recommend corticosteroid administration from week 34 (Fig. 3 and Supplementary Table 2).
All panellists agreed not to recommend an additional dose of corticosteroids between 34 and 36 weeks of gestation in women at high risk of preterm delivery if a first course had been administered before 34 weeks. Most panellists agreed that antenatal corticosteroid administration offers respiratory benefits (76%). The reasons for not recommending corticosteroid administration between 34 and 36 weeks of gestation included an increased risk of hypoglycaemia in the newborn (79%) and a potential deleterious impact on long-term neurodevelopmental outcomes (83%) (Fig. 3).
DiagnosisRegarding RDS diagnosis, all panellists (100%) considered that the lung ultrasound (LUS) offers advantages over the chest radiograph and that it should be performed within 2 h of birth (100%) (Fig. 4 and Supplementary Table 2).
Most panellists considered that the differential diagnosis of RDS should include transient tachypnoea of the newborn (TTNB) (97%), mild respiratory distress (100%), congenital pneumonia (93%) and primary pulmonary hypertension (PPH) (76%) (Fig. 4). Moreover, cardiologists should evaluate late preterm neonates with suspected RDS and an unfavourable clinical course to rule out PPH (Fig. 4).
TreatmentDecision to administer surfactantMost panellists (93%) agreed that studies were needed to determine the benefits and harms, clinical profile and methods of surfactant administration in moderate/late preterm neonates (Supplementary Table 2). The factors identified as most important in making the decision whether to administer surfactant were: FiO2 level (100% of panellists), severity of respiratory distress (100%), LUS score (100%), respiratory acidosis (93%), previous mean airway pressure (93%) and time elapsed from birth to diagnosis (97%) (Supplementary Table 2). Panellists recommended surfactant administration within 2–6 h of birth in infants with a diagnosis of RDS (86%) if the previous mean airway pressure was 6 mmH2O or greater (83%) and/or the LUS score is 6 or greater (93%) (Fig. 5 and Supplementary Table 2).
Most panellists (83%) would recommend surfactant administration in moderate/late preterm neonates with respiratory distress and an unfavourable clinical course, even when the diagnosis of RDS is uncertain (Supplementary Table 2). Moreover, most panellists would administer surfactant for other conditions, including meconium aspiration syndrome (90%) or congenital pneumonia (83%) (Fig. 5). However, most would not administer surfactant in cases of congenital diaphragmatic hernia, pulmonary hypoplasia or TTNB (Supplementary Table 2).
Administration technique and potential complicationsRegarding the method of surfactant administration, all respondents would use the MIST technique (Fig. 5 and Supplementary Table 2). Panellists agreed that the recommended initial dose of surfactant for a late preterm neonate is 200 mg/kg (97%). No consensus was reached on caffeine administration before surfactant administration via MIST (Fig. 5).
Benefits and harms of surfactant administration in these patientsAll panellists agreed that surfactant administration could reduce the degree of respiratory distress, improve respiratory parameters, reduce the duration and the need for invasive mechanical ventilation (MV) and shorten the length of stay in the intensive care unit (ICU) and/or the hospital. Moreover, most agreed that surfactant administration could lower the risk of mortality (79%), decrease the need for a referral from a lower-level to a tertiary care hospital (79%) and reduce the incidence of some of the morbidities typically associated with prematurity (83%). On the other hand, panellists agreed that not administering surfactant to these patients would not increase the risk of necrotizing enterocolitis (97%), retinopathy of prematurity (100%) or intracranial haemorrhage (93%) (Supplementary Table 2).
Sedation measures for MISTRegarding sedation, most participants agreed on the use of nonpharmacological methods as the initial approach, such as the administration of sucrose (97%) or breastfeeding (72%) 2 min before the procedure, both accompanied by swaddling (Fig. 6 and Supplementary Table 2). In the case that nonpharmacological measures are ineffective, 3 out of 4 respondents agreed on using an opioid as the first-line sedation agent for surfactant administration via MIST (Fig. 6).
Sequelae and follow-upAll respondents agreed that moderate/late preterm neonates are at higher risk of neonatal morbidity and mortality compared to term neonates, particularly respiratory problems (Fig. 7 and Supplementary Table 2). Most neonatologists (90%) agreed that all late preterm neonates with perinatal risk factors or complications in the neonatal period need more specialized monitoring in hospital-based follow-up visits. Moreover, there was consensus that the prevalence of immediate and long-term respiratory disease is higher in moderate/late preterm neonates compared to term neonates. Lastly, most respondents agreed that in moderate/late preterm neonates, a history of chorioamnionitis and absence of breastfeeding are risk factors for developing asthma or pulmonary disease in the long term (Fig. 7).
Future trendsAll respondents agreed that there was a lack of large-scope longitudinal population-based studies identifying factors associated with an increased risk of adverse outcomes in moderate/late preterm neonates and pre- and postnatal risk factors that increase the likelihood of impaired lung function in school age (Supplementary Table 2).
DiscussionAlthough a significant body of evidence on the management of RDS in preterm neonates has been gathered over the years, guidelines chiefly focus on very preterm infants (<32 weeks of gestation).15 The results of this project address a current gap in the field, providing recommendations for both the diagnosis of RDS and its management in moderate/late preterm infants that can guide decision-making concerning surfactant administration.
In preterm infants of lower gestational age (<32 weeks of gestation), antenatal corticosteroid administration provides respiratory benefits and reduces the risk of mortality and other morbidity (intraventricular haemorrhage, necrotising enterocolitis).18 On the other hand, the benefit/harm balance of antenatal corticosteroid administration in late preterm infants is still unclear. In any case, antenatal corticosteroid administration has been associated with an increased risk of hypoglycaemia19–21 and there are concerns about its long-term effects on neurodevelopment. Several studies have demonstrated an increased risk of neurocognitive and behavioural abnormalities.22,23
Furthermore, there is substantial heterogeneity among international recommendations; the American College of Obstetricians and Gynecologists (ACOG) considers its administration in mothers at high risk of preterm delivery within 7 days between 34 and 36 weeks,24 while in Europe the indication is more controversial.15 There was consensus regarding the lack of sufficient evidence to adequately establish the benefit/harm balance in the administration of antenatal corticosteroids. Furthermore, there was consensus among the panellists to not recommend an additional dose of corticosteroids between 34 and 36 weeks of gestation in the case of high risk of premature delivery, nor between 34 and 34+6 weeks of gestation.
In recent years, the LUS has been integrated as a useful technique in the diagnosis and management of RDS. For diagnosis of RDS, it is preferable to perform a LUS rather than an X-ray because the former is highly sensitive and avoids exposure to radiation.25 When it comes to RDS management, it is important to determine the need for treatment, specifically surfactant administration. When performed shortly after birth, LUS can predict the respiratory support/surfactant treatment needs of late preterm infants.26 In this regard, all panellists agreed on performing a LUS within 2 h of birth, highlighting the importance of early diagnosis to achieve maximum benefit. The lack of evidence regarding the LUS score to guide surfactant administration in late preterm infants led the panellists to establish a cutoff of 6 h during the Delphi process. However, new evidence has emerged since the questionnaire was developed, and De Luca et al demonstrated that a LUS score higher than 8 is associated with the highest global accuracy, supporting its use to guide surfactant administration. The same study showed that a LUS score of 4 or lower is associated with the highest sensitivity, indicating that the need for surfactant is unlikely in this group.27
No specific guidelines on surfactant administration in late preterm infants are available; however, several studies have shown that it is safe, improves respiratory outcomes and decreases mortality.28,29 Overall, there is a lack of studies on surfactant administration in late preterm infants. However, an ongoing trial, SURFON (SURFactant Or Not), is investigating the early use of surfactant in late preterm infants.30 In the present study, most panellists recommended surfactant administration in moderate/late neonates, although there were doubts regarding the diagnosis of RDS. In this regard, when panellists were asked about the administration of surfactant for other pathologies, they mostly agreed on its use for conditions such as congenital pneumonia and meconium aspiration syndrome. In contrast, a survey on the use of surfactants in late preterm infants among Belgian neonatologists highlighted the use of surfactants for RDS and meconium aspiration syndrome. Still, there was less unanimity regarding its use in transient tachypnoea of the newborn and congenital pneumonia.31
Although surfactant administration is the main treatment for RDS, the method of administration is still under debate. Minimally invasive techniques (less invasive surfactant administration [LISA] or minimally invasive surfactant therapy [MIST]) also offer advantages in moderate/late preterm infants.15 They achieve respiratory improvement with few adverse effects, a significant reduction in exposure to mechanical ventilation, a reduction in the need for transfer to a tertiary care facility,32 a non-significant reduction in neonatal ICU and hospital lengths of stay33 and a decrease in the risk of pneumothorax.34 In this Delphi study, there was unanimity in favour of the use of MIST, preferably with devices specifically designed for the purpose. There was broad consensus about the 200 mg/kg dosage, which was consistent with the most recent recommendations.15 There was no consensus regarding the administration of caffeine prior to administration of surfactant.
There is considerable controversy in the literature regarding the need for pharmacological sedation/analgesia during MIST. There was consensus among panellists in favour of sedation for late preterm infants, with sucrose preferred over breast milk in the case of nonpharmacological sedation. In this regard, in a recent survey conducted in Spain, all participating hospitals (100%) reported the use of sucrose.35 Notably, up to 70% of Spanish hospitals reported using pharmacological sedation/analgesia before the procedure,35 compared to 52% in Europe.36 There is also controversy in the literature regarding the appropriate type of drug and dose in the case of pharmacological sedation.37 In the present consensus, most panellist would prescribe sedation, prioritizing morphine derivatives (76%). In this line, several surveys have shown that the agents most frequently used for sedation are opioids (23%–63%), followed by propofol (5%–23%), benzodiazepines (5%–23%), ketamine (9%), and muscle relaxants (9%).35,36
Regarding follow-up, there is evidence that moderate and late preterm infants are at risk of changes in neurodevelopment.38–40 In line with this, panellists agreed in recommending long-term follow-up. Studies are needed to determine the risk factors for adverse outcomes to identify children who require specialised follow-up. The long-term sequelae in late preterm infants include impaired pulmonary function in late childhood and adolescence.41 An ongoing longitudinal prospective study (LaPrem) is evaluating the impact of preterm birth (32–36 weeks of gestation) on neurodevelopment, brain development and respiratory health in late childhood.10 In parallel, the SEN32-36 working group of the Spanish Society of Neonatology is conducting a nationwide study with the same aim.
The “small” size of the panel could be considered a limitation, although the ideal number of panellists for a Delphi consensus has not been established. In addition, small panels yield reliable criteria when they are composed of highly qualified experts (as is the case of the present study). On the other hand, we cannot overlook the intrinsic limitations of the Delphi design, for instance, that the results derive from opinions without the analysis of retrospective or prospective data. Furthermore, studies with statistical power focused specifically on moderate/late infants will help elucidate the best management for these patients and identify prognostic factors. Moreover, in the future, performance of studies to assess the role of antenatal corticosteroids in late preterm infants would be of utmost interest, and further research is needed to identify pre- and postnatal risk factors for lung function impairment in late childhood.
In the absence of conclusive data regarding prevention, diagnosis, treatment, and follow-up of RDS in moderate/late preterm infants, and given that most clinical decision rules have been extrapolated from studies performed in preterm infants born before 32 weeks, the present study can help guide decision-making, at least until more scientific evidence becomes available.
FundingChiesi Españaprovided financial assistance for the development and performance of the study, methodological support and medical writing.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank all the experts who participated in the Delphi survey, Jemina Moreto, Ph.D., from Trialance, for the writing support, and Carmen González, from GOT IT Consulting, for the methodological support.