To study the epidemiology, clinical features, diagnosis, therapeutic management, and outcome of non-tuberculous mycobacterial lymphadenitis in a paediatric population of Aragón (Spain).
Material and methodsA retrospective study was conducted on patients under 15 years-old diagnosed with non-tuberculous mycobacterial lymphadenitis between the years 2000 and 2015. Inclusion criteria: patients with lymphadenitis and positive culture. Quantitative values are shown as mean, rank, and standard deviation, and qualitative data as frequencies.
ResultsTwenty-seven cases were registered, with a mean age of presentation of 39.9 months (range 10 months–8 years). The mean time between the symptoms onset and first consultation was 1.7±1.1 months. The most frequent location was sub-maxilar in 17/27 cases (63%), on the right side in 59.3%, and size 2.96±1.26cm. Fistulae were observed in 16/27 cases. Tuberculin test was greater than 10mm in 7/24 (29.1%). Microbiological cultures were positive for Mycobacterium avium in 14/27 (51.9%), Mycobacterium intracellulare 3/27 (11.1%), and Mycobacterium lentiflavum 3/27 (11.1%). Combined treatment of antibiotics and surgery was given in 16/27 cases (59.8%), medical treatment only in 7/27 (25.9%), and surgical exeresis alone in 4/27 (14.8%). Two patients required a new surgery, and one showed severe neutropenia secondary to rifabutin. Only one case (3.7%) suffered from temporary facial palsy as sequel.
ConclusionsThe most frequent treatment was the combination of antibiotics and surgery. Delay in diagnosis seemed to be responsible for the limited number of exeresis as first option, only one for every seven patients.
Estudiar la epidemiología, las manifestaciones clínicas, el manejo diagnóstico-terapéutico y la evolución de las linfadenitis por micobacterias no tuberculosas en la población pediátrica de Aragón.
Material y métodosEstudio retrospectivo de pacientes menores de 15 años diagnosticados de linfadenitis por micobacteria no tuberculosa entre 2000 y 2015. Criterios de inclusión: pacientes con linfadenitis y cultivo positivo. Los resultados se expresan como medias, rango y desviación típica para las variables cuantitativas, y porcentajes para las cualitativas.
ResultadosSe detectan 27 casos, edad media de presentación 39,9 meses (rango 10 meses-8 años). El tiempo desde inicio de los síntomas hasta la primera consulta especializada es 1,7±1,1 meses. La localización más frecuente es submaxilar en 17/27 casos (63%), lado derecho en el 59,3%, con tamaño de 2,96±1,26cm. Solo 16/27 presentan fistulización. Prueba de tuberculina superior a 10mm en 7/24 (29,1%). El cultivo es positivo para Mycobacterium avium en 14/27 (51,9%), Mycobacterium intracellulare 3/27 (11,1%), Mycobacterium lentiflavum 3/27 (11,1%). El 92,6% (23/27) es tratado inicialmente con amoxicilina-clavulánico. La combinación de antibióticos y cirugía se aplica en 16/27 casos (59,3%), solo antibioterapia 7/27 (25,9%) y únicamente exéresis 4/27 (14,8%). Dos pacientes precisan reintervención y un caso desarrolla neutropenia grave secundaria a rifabutina. Solo un caso (3,7%) presenta parálisis facial transitoria como secuela.
ConclusionesLa combinación de antibioterapia y cirugía es el tratamiento más frecuente. El retraso en el diagnóstico hace que la exéresis como primera opción terapéutica se realice únicamente en uno de cada 7 pacientes.
Cervical lymphadenitis is the most frequent manifestation of infection by nontuberculous mycobacteria (NTM) in immunocompetent children. It amounts to 10%–20% of cervical, submandibular and preauricular lymphadenitis cases in early childhood. It affects children aged 1–5 years and is rare after age 10 years, unlike tuberculous lymphadenitis.1
Nontuberculous mycobacteria are found in water, soil, milk, animals (mainly birds) and health care equipment. Infection of the cervical lymph nodes follows inhalation, inoculation or ingestion of contaminated material in 90% of cases. The development of a unilateral, painless isolated mass in the submaxillary or cervical region is the most frequent clinical manifestation. The natural history of the disease involves a progression of the adenopathy, with the overlying skin taking a violaceous hue, fistula formation in three to four months, and a chronic indolent course that ends with skin scarring in 12–18 months.2
In recent years, its incidence has increased both in Spain and worldwide.3–5 Until the mid-1990s, the incidence was of 1.2cases/year based on data from a study conducted in a tertiary hospital of the Autonomous Community of Madrid. At present, in this same hospital, it is of 5.25cases/year.3 In countries such as the Netherlands or Australia, the incidence reaches up to one case per 100000 inhabitants.4,5
However, most of the data published in the literature is from case series, and no conclusive controlled clinical trials have been conducted to assess the efficacy and safety of the different approaches to its management. Although complete excision of the affected lymph node is currently the gold standard, this is difficult to implement due to delays in diagnosis. However, other alternatives such as close observation and pharmacological treatment alone or combined with surgery are management options that seem to achieve similar outcomes.6
Materials and methodsWe conducted a multicentre, retrospective observational study between 2001 and 2015 in patients aged less than 15 years with a diagnosis of nontuberculous mycobacterial lymphadenitis (NTMLA) recorded in the database of the Microbiology Laboratory of the Hospital Universitario Miguel Servet de Zaragoza (HIUMS), the referral hospital in our autonomous community. The criteria for inclusion in the study were the following:
- a.
Previously healthy individual with no underlying chronic disease.
- b.
Clinical manifestations compatible with acute, subacute or chronic lymphadenitis.
- c.
Microbiological diagnosis of infection by NTM: positive culture.
We reviewed data for clinical manifestations, diagnostic tests, treatments and outcomes found in patient health records. We assessed the size of the lymphadenopathy using the maximum diameter in centimetres.
Samples for microbiological testing were obtained by fine-needle aspiration (FNA) or biopsy. Testing for the presence of acid-fast bacilli was performed by the customary methods: Ziehl–Neelsen and auramine staining. Samples were cultured in solid and liquid media until 2007, and in liquid media alone thereafter. The mycobacterial species was identified by means of the GenoType CM/AS method (Hain Lifescience GmbH, Nehren, Germany), and in 2015 by means of the Matrix Assisted Laser Desorption Ionisation Time-of-flight method (MALDI-TOF; Bruker Daltonik GmbH, Bremen, Germany).
Six of the seven hospitals that offer paediatric services in the autonomous community of Aragón participated in the study. All hospitals follow the same protocol for the management of NTMLA. These patients are referred to and managed by the outpatient paediatric surgery and paediatric infectious disease clinics of the HIUMS, which is the referral hospital.
We considered that the lymphadenopathy had been cured when the following criteria were met: diameter of less than 1cm, total closure of the overlying skin, and absence of local recurrence. The sequelae under study were unsightly scars and cranial nerve involvement (transient or permanent). Only one case was lost to followup.
We have expressed the results as mean, range and standard deviation for quantitative variables, and in the form of percentages for qualitative variables.
ResultsWe included a total of 27 patients (female, 14 [51.9%]) that were previously healthy and had not been exposed to tuberculosis (TB) disease. With the exception of a patient of Maghrebi descent and one from Sub-Saharan descent, both of whom had been residing in Spain for more than six months, all patients were Spanish nationals. The mean age of onset was 39.9 months (range, 10 months–8 years) and the incidence was 1.68cases/year. The incidence peaked with five cases in 2012.
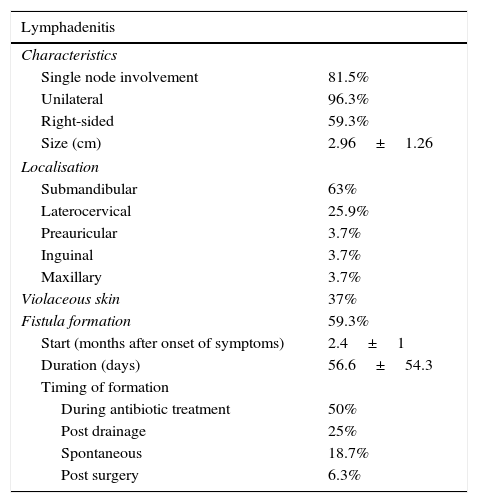
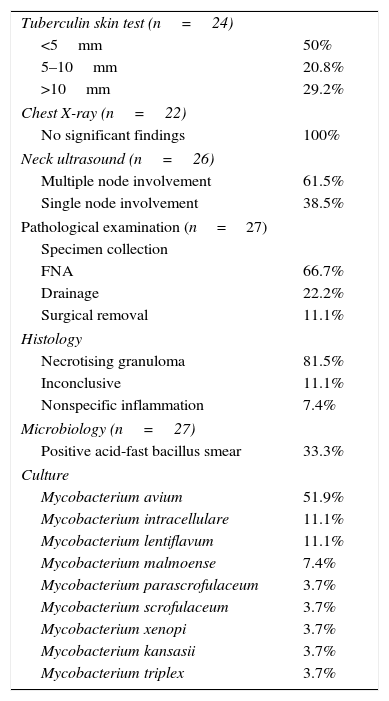
Of all patients, 96.3% reported having no associated symptoms. Only one patient presented with fever that developed concurrently with the lymphadenopathy. Table 1 summarises the rest of the clinical characteristics. All patients underwent routine testing including a complete blood count, measurement of C reactive protein (CRP), erythrocyte sedimentation rate, blood chemistry panel including transaminase and LDH levels, and antibody tests for cytomegalovirus, Epstein–Barr virus and toxoplasmosis, all of which were normal. Table 2 shows the rest of the diagnostic tests that were performed.
Nontuberculous bacterial lymphadenitis: clinical characteristics.
| Lymphadenitis | |
|---|---|
| Characteristics | |
| Single node involvement | 81.5% |
| Unilateral | 96.3% |
| Right-sided | 59.3% |
| Size (cm) | 2.96±1.26 |
| Localisation | |
| Submandibular | 63% |
| Laterocervical | 25.9% |
| Preauricular | 3.7% |
| Inguinal | 3.7% |
| Maxillary | 3.7% |
| Violaceous skin | 37% |
| Fistula formation | 59.3% |
| Start (months after onset of symptoms) | 2.4±1 |
| Duration (days) | 56.6±54.3 |
| Timing of formation | |
| During antibiotic treatment | 50% |
| Post drainage | 25% |
| Spontaneous | 18.7% |
| Post surgery | 6.3% |
Nontuberculous mycobacterial lymphadenitis: diagnostic tests.
| Tuberculin skin test (n=24) | |
| <5mm | 50% |
| 5–10mm | 20.8% |
| >10mm | 29.2% |
| Chest X-ray (n=22) | |
| No significant findings | 100% |
| Neck ultrasound (n=26) | |
| Multiple node involvement | 61.5% |
| Single node involvement | 38.5% |
| Pathological examination (n=27) | |
| Specimen collection | |
| FNA | 66.7% |
| Drainage | 22.2% |
| Surgical removal | 11.1% |
| Histology | |
| Necrotising granuloma | 81.5% |
| Inconclusive | 11.1% |
| Nonspecific inflammation | 7.4% |
| Microbiology (n=27) | |
| Positive acid-fast bacillus smear | 33.3% |
| Culture | |
| Mycobacterium avium | 51.9% |
| Mycobacterium intracellulare | 11.1% |
| Mycobacterium lentiflavum | 11.1% |
| Mycobacterium malmoense | 7.4% |
| Mycobacterium parascrofulaceum | 3.7% |
| Mycobacterium scrofulaceum | 3.7% |
| Mycobacterium xenopi | 3.7% |
| Mycobacterium kansasii | 3.7% |
| Mycobacterium triplex | 3.7% |
The mean time elapsed to the first related visit at a health care centre or hospital emergency department was 8.93 days (range, 0–35), and the mean time elapsed from onset until the patient was seen by a specialist in infectious disease or paediatric surgery at the referral hospital was 1.69±1.09 months.
The initial treatment in most patients (23/27) was broad-spectrum oral antibiotherapy with amoxicillin–clavulanic acid, initiated at a mean of 10.91 days from the onset of symptoms (range, 0–66 days) and with a mean duration of 9.26±2.02 days. Surgical excision was the initial treatment in only one case, a girl in whom two lymphadenopathies (from which NTM were subsequently isolated) were resected in the context of surgery for adenophlegmon by the maxillofacial surgery department. Antibiotic therapy was recommended, but the patient refused this option, so she was monitored periodically until the lesion resolved. Another patient received treatment specific to NTM from the outset due to high clinical suspicion, while the two remaining patients were treated with antituberculous drugs and drainage of the lymphadenopathy, respectively, before NTMLA was suspected.
Later on, while awaiting the results of culture, patients received specific treatment for NTMLA. Three patients underwent elective surgical excision, while 85.2% (23/27) of patients received oral antibiotherapy. In these patients, antibiotherapy was initiated 2.24±1.44 months from the onset of symptoms, and lasted 5.76±1.77 months. Out of the 23 patients, 16 required surgical excision after treatment completion, and nine continued antibiotic therapy for a mean additional 2.14 months. None of the patients were managed with watchful waiting.
When it came to antibiotherapy, of the 23 patients that received it, 30.4% (7/23) required at least one change of the drugs used. The most frequent reason for changes was lack of response (71.4%), and the other reasons were the results of antibiotic sensitivity testing and the development of neutropaenia secondary to administration of rifabutin. The most frequent antibiotic combination was clarithromycin with rifabutin (33%), followed by clarithromycin with rifampicin (20%) and azithromycin with rifabutin (13.3%).
Therefore, the most frequently used treatment was a combination of antibiotherapy and surgery, implemented in 59.3% (16/27) of our case series. Pharmacological treatment alone was used in 25.9% (7/27) of the patients, while surgery as the sole treatment was performed in 14.8% (4/27).
The mean duration of followup was 13.13±4.9 months. Two patients required surgical reintervention: one due to persisting lymphadenopathy despite combined treatment, and another due to recurrence of lymphadenitis twelve months later. Surgical excision was recommended to one patient after completion of antibiotherapy, but the patient was lost to followup, while the parents of another patient refused antibiotic treatment after surgical excision, with complete resolution of the lymphadenopathy observed in subsequent follow-up visits. The sequelae observed in our case series were significant hypertrophic scarring in 18.5% (5/27), and transient palsy of the marginal mandibular branch of the left facial nerve in only one patient (3%).
DiscussionSeveral studies published recently suggest that the incidence of infection by NTM has increased in recent years. However, the data in our case series (1.68cases/year) show a lower incidence than the one found in recent years in a tertiary hospital of the Autonomous Community of Madrid of similar characteristics to our hospital (5.25cases/year).3 Some authors attribute this increase to the improved control of TB disease and the reduction in the number of vaccinations against TB in developed countries. The control of TB disease has led to an increased susceptibility to NTM, which seems to be the factor that contributes most to the inaccurately inflated prevalences of TB disease found in some regions in Spain.7 It is estimated that between 20% and 60% of children with a positive tuberculin skin test result with no risk factors for TB could be infected by NTM.8 Vaccination could protect against several NTM species, something that has been demonstrated in animal models.9–11
Cervical lymphadenitis is the most frequent manifestation of infection by NTM in immunocompetent children. The overlaying skin usually acquires a violaceous hue when it reaches stage III of the classification proposed by Penn et al.12 In our case series, 63% of the patients did not present with this change in the first visit, and it is difficult to determine whether subsequent changes were due to the natural course of disease or produced by diagnostic tests (drainage or FNA). The absence of accompanying symptoms is another characteristic feature, although a small number of cases have been described in the literature that presented with isolated fever in the initial stages.1
Chest radiography is recommended in every case, especially under the circumstances listed in Table 3, while neck ultrasound is the imaging test of choice due to its accessibility and safety.3 In our series, chest X-rays were performed in 81.5% of the patients, even though they did not meet the indications recommended in the literature. Samples for histopathological and microbiological analysis can be obtained by three different methods: FNA, drainage and excision. We ought to underscore that FNA (the most frequently used technique in our study) is considered a quick and easy test in facilities with experienced staff,3 but that increases the risk of fistula formation otherwise.13 The most frequent histological finding is the presence of granulomas,14 consistent with our study, in which they were found in 66.67% of patients. Other possible findings are necrosis, caseous or not, or nonspecific inflammation. The prevalence of positive results for acid-fast bacilli in our series was below the 50% to 60% reported in the literature.15
The definitive diagnosis requires the isolation1 or detection by PCR16 of NTM. The sensitivity of culture depends on the method used for sample collection. Fine-needle aspiration has a lower yield, as the concentration of mycobacteria can be low.17 In general, based on different studies, cultures are positive in 20% to 75% of patients.18,19 We cannot compare these figures with our study, as it only included patients with compatible clinical manifestations and positive culture. Direct molecular detection by PCR, a method that is available in a few health care facilities, allows the early identification of the mycobacterium and ruling out of tuberculous lymphadenitis, with a specificity greater than 95% and a sensitivity that varies between 50% and 100% depending on the bacterial load of the sample.3 In our hospital, PCR for detection of Mycobacterium tuberculosis complex is performed first. If the result is negative, PCR for NTM is deferred until the results of culture are received.
The most frequent aetiologic agent in most of Europe, the United States and Australia is Mycobacterium avium-intracellulare complex (70%–80%), followed until a few years ago by Mycobacterium scrofulaceum (10%–20%) and Mycobacterium kansasii (5%).20 However, in the past five years isolation of Mycobacterium lentiflavum has increased to the point of becoming the second most frequent in Spanish and Australian case series.1,2 The data in our series are consistent with this.
Lymphadenitis due to M. tuberculosis, or tuberculous lymphadenitis (TLA), is the main differential diagnosis. It accounts for 10%–20% of cases of lymphadenitis due to mycobacteria in the paediatric population. It typically affects children aged more than 10 years and presents with systemic manifestations in posterior cervical or axillary locations. Thus, a tuberculin skin test should be performed in any child presenting with subacute or chronic lymphadenitis. However, the size of induration cannot be used to rule out NTMLA, and it is advised that the diagnosis be supported by additional clinical and epidemiological data. The tuberculin skin test results in our series were consistent with those found in the studies by Cavalho et al. and Lindeboom et al., in which 20%–65% of patients developed indurations of more than 5mm, although the percentage of patients in our study with indurations of more than 10mm was lower (29.2% compared to 50%).21,22 Chest radiography may be useful, as it finds abnormalities in 37%–56% of TLA cases and only 8.5% of NTMLA cases.22 If the patient has no risk factors but the results of histological examination are compatible and/or the results of acid-fast staining are positive, a working diagnosis of TB should be assumed until disproven, and treatment initiated,17,23 as occurred in one of our patients. The differential diagnosis must also include other possible causes of granulomatous lymphadenitis, such as toxoplasmosis or cat-scratch disease.3
Early surgical removal is the gold standard of treatment currently recommended, preferably in the first month from onset.24 It is associated with a higher cure rate, lower incidence of recurrence and better aesthetic outcomes, and provides better specimens for pathological and microbiological analysis.17 If not performed within this window, spontaneous fistulisation may occur, which increases morbidity and the complexity of surgical intervention.14 The risk of reintervention is higher—a difference that is statistically significant—if drainage or curettage are chosen as the initial approach.25 In our series, we found a high incidence of fistula formation, probably due to this fact. However, the frequency of reintervention was very low considering that a substantial percentage of patients underwent drainage for specimen collection. The most frequent postoperative complication is transient facial palsy, which develops in 2% to 17% of cases.26 The most frequent sequela in our patients was hypertrophic scarring, which affected aesthetic outcomes.
Pharmacological treatment and watchful waiting are the alternatives to surgery. Concurrent use of several drugs is recommended to prevent drug resistance, including a macrolide (azithromycin or clarithromycin) in combination with rifabutin/rifampicin6 or ciprofloxacin or ethambutol.3,27 At present, there is no consensus on the duration of antibiotic treatment, but a minimum of three to six months is recommended. For species other than M. avium complex, such as M. kansasii or M. lentiflavum, broader coverage with rifabutin, ethambutol, isoniazid and pyridoxine is recommended due to the prevalence of drug resistance, while other species, such as Mycobacterium fortuitum, are very sensitive to advanced-generation macrolides and/or quinolones.28
The low incidence of NTMLA has precluded the development of controlled clinical trials comparing medical and surgical treatment in children. Small-scale studies, such as those conducted by Hazra et al. and Berger et al., have found a greater efficacy of macrolide therapy compared to surgery.29,30 In a study by Luong et al., 45 out of 55 (81.8%) children with NTMLA received antibiotics as the initial treatment; 67% of these 45 did not require surgical excision, and lymphadenitis starting to regress within two months in 77% of them (23 patients).31 The largest study, conducted by Lindeboom et al., randomly assigned a total of 100 children with NTMLA to groups treated with clarithromycin combined with rifabutin, watchful waiting or surgery. The cure rate was 96% in the surgery group compared to 66% in the antibiotherapy group. The mean time to resolution did not differ significantly between the antibiotherapy and the watchful waiting groups.32 Few observational studies have adopted a watchful waiting approach. Zeharia et al. found that out of a total of 92 Israeli children with NTMLA managed with observation alone, 71% experienced full resolution of lymphadenitis at six months and 100% at nine to twelve months.33 A recently published literature review by Zimmermann et al. compared these and other studies performed to date.6 The review concluded that large, well-designed randomised controlled trials comparing the three treatment modalities and better diagnostic tests to enable studies without the need for surgical intervention are needed, and that until such evidence becomes available, treatment options should be carefully considered on an individual basis weighing potential risks against benefits.
Only one of our patients developed complications secondary to antibiotic treatment. It was a boy aged 2 years with severe neutropaenia caused by rifabutin, which resolved after switching antibiotics,34 a response that, while rare, has been described in clinical trials with healthy subjects.35
We want to highlight, having analysed the course of these cases over time, that fulfilling the timelines recommended in the literature for offering surgical excision as the first-line treatment poses significant challenges. The protocols in our hospital and national protocols for Spain, as well as international guidelines, recommend initiation of empirical antibiotherapy by the oral route in cases of unilateral lymphadenopathy with no significant warning signs and of idiopathic or postinfectious aetiology, with observation in the following three to four weeks. If the lesion does not resolve within this time frame, diagnostic tests should be performed.36–38 In our experience, surgical excision in most patients takes place after initiation of anti-NTM treatment, and the combination of these two modalities results in resolution rates similar to those reported in the literature, as was the case with the sequelae and complications.
There are limitations to our study, as it only included cases with positive NTM culture results. Thus, the study did not include cases that, while having negative culture results, had compatible clinical manifestations and received NTM-specific treatment, which leads to an underestimation of the prevalence of this pathology in our region.
To conclude, our experience was consistent with the literature, with similar findings except in relation to the timing of surgical excision as the definitive treatment. The scarcity of studies and clinical trials hinders adherence to a specific action protocol. We think that interdisciplinary cooperation (between paediatric infectious disease specialists, microbiologists and surgeons) in close communication with primary care providers is essential to the early and effective management of these patients.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Ruiz del Olmo Izuzquiza I. Linfadenitis por micobacterias no tuberculosas: experiencia de 15 años. 2017;86:115–121.