The use of levamisole in the management of nephrotic syndrome started soon after the authorisation of steroid therapy.1 It is an antihelminthic agent with immunomodulatory properties that are not well understood, and without immunosuppressive effects, unlike the rest of the drugs used in these patients.2 On the other hand, it is the least toxic and least expensive drug.3 In 2004, it was withdrawn from commercial markets due to the lack of clear indications and its infrequent use in humans.4 It has proven effective in some patients with steroid-dependent primary nephrotic syndrome as a steroid-sparing agent.4
We conducted a retrospective descriptive study on the population of patients with nephrotic syndrome and high-dose steroid dependency given levamisole at the paediatric nephrology unit of our hospital between January 1, 2000 and December 31, 2017. Of the 104 cases of nephrotic syndrome we reviewed, we excluded 38% due to missing data. Levamisole was given to patients with steroid-dependent nephrotic syndrome treated with high-dose prednisone (> 0.5 mg/kg/48 h). In some cases, this followed treatment with oral cyclophosphamide and in others it preceded administration of cyclophosphamide (second step treatment). A histopathological examination was not performed, as this diagnostic test is performed prior to initiation of the third step of treatment. We classified patient response to levamisole into 2 categories: “complete” when patients experienced no recurrences over at least 2 years of treatment, and “partial” when patients experienced 2 or fewer recurrences in 1 year, allowing discontinuation of steroid therapy. The drug was compounded in the prescribed dose in gel cap form at the hospital’s pharmacy. All patients received the standard dose of 2.5 mg/kg of body weight every 48 h, administered orally.
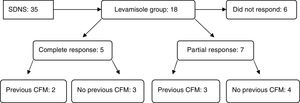
A total of 18 patients received levamisole, 10 girls and 8 boys, aged 2–6 years. Of these 18 patients, 12 (66.6%) responded to the treatment: 5 exhibited complete remission (27.7%) and 7 (38.8%) partial remission (Fig. 1). Treatment was discontinued after 2 years in patients that responded, but it had to be reintroduced in 7 out of the 12 due to recurrence. The mean duration of treatment was 4.3 years. One patient with levamisole dependence continued treatment through his transition to adult care. As for adverse reactions, only 2 patients developed transient urticaria attributable to levamisole that did not require discontinuation of the drug.
In recent years, few studies have assessed the efficacy of levamisole. Furthermore, it is inexplicably absent from most guidelines and clinical management protocols, except in France, and thus not assigned a specific timing in the stepwise management of nephrotic syndrome, unlike the rest of the treatment options.5 The few studies that have been published had study periods of at most 1 year, and usually did not have a prospective design, except for an interesting multicentre clinical trial published in 2018 with a 1-year follow-up.3 In this trial, 26% of patients treated with levamisole had not experience recurrences at 1 year, a percentage similar to the one found in our sample at 2 years (27.7%). Another study involved administration of higher doses (double) when patients did not respond to the standard dose, which had positive results.6
When it comes to adverse reactions, in addition to urticaria, there have been reports of mild to moderate neutropenia, which did not occur in any of the patients in our sample.3
In Spain, levamisole is available under the regulation applied to foreign drugs. At present, authorised drugs that are used off-label are requested according to this protocol and do not require express authorization from the Agencia Española de Medicamentos y Productos Sanitarios (Spanish Agency of Medicines and Medical Devices) (Royal Decree 1015/2009 of June 19).
Levamisole is a valid therapeutic option whose efficacy has not been properly evaluated and that is frequently overlooked in nephrotic syndrome treatment protocols. For this reason, we propose performance of clinical trials of levamisole with longer durations of follow-up to assess its performance and thus establish the place that this drug deserves, in our opinion, in treatment protocols.
Please cite this article as: Soto LG, Romera PM, Andrade JEV, Llorente MAG, Fernández PdD. Experiencia con levamisole en el tratamiento del síndrome nefrótico primario corticodependiente. An Pediatr (Barc). 2020;92:168–169.