The aim of this study was to assess the safety and efficacy of ledipasvir/sofosbuvir combination in chronic Hepatitis C Virus (HCV) genotype 1 and 4 infection in paediatric patients.
MethodsEligible patients to be treated with ledipasvir/sofosbuvir were patients from 6 to 18 years old with a chronic HCV genotype 1 or 4 infection. The duration and doses of antiviral drugs were changed depending on patient age, fibrosis stage, and PEGylated interferon+ribavirin experience status. The primary efficacy endpoint was the percentage of patients with a sustained virological response 12 weeks posttreatment.
ResultsA total of nine patients (7 males) with a median age of 14.8 years (8.48–17.91) were treated with ledipasvir/sofosbuvir combination. Five patients received previous treatment with PEGylated interferon+ribavirin during a median of 8.5 months (3–12 months). Eight patients had some degree of fibrosis (1 patient presented with F1, three patients F2, 2 patients F3, and 2 patients F4). The median pre-treatment viral load was 6.2log [5.9–6.8] with the HCV RNA becoming negative six weeks after starting the treatment in 100% of the patients. All patients maintained a sustained viral response at 12 weeks. Three patients (33.3%) had some type of adverse effect (2 headache and one oral thrush). The median posttreatment follow-up was 24 weeks (12–104).
ConclusionsTreatment with ledipasvir/sofosbuvir in paediatric patients with chronic HCV infection genotype 1 and 4 is safe and effective with SVR12 and similar to those reported in adults.
El objetivo del estudio fue evaluar la seguridad y la eficacia de la combinación de ledipasvir/sofosbuvir en la infección crónica por el genotipo 1 y 4 del virus de la hepatitis C (VHC) en pacientes pediátricos.
MétodosSe incluyó a pacientes de entre 6 y 18 años. La duración y la dosis de los fármacos antivirales se administraron según la edad del paciente, el estadio de fibrosis y los tratamientos previos con interferón pegilado y ribavirina. La variable principal de eficacia fue el porcentaje de pacientes con una respuesta virológica sostenida 12 semanas (RVS12) después del tratamiento.
ResultadosNueve pacientes con una mediana de edad de 14,8 años (8,48-17,91) fueron tratados con combinación de ledipasvir/sofosbuvir. Cinco pacientes habían recibido previamente tratamiento con interferón pegilado+ribavirina. Ocho pacientes tenían algún grado de fibrosis. La mediana de la carga viral previa al tratamiento fue de 6,2 log (5,9-6,8) con negativización del ARN del VHC 6 semanas después de comenzar el tratamiento en el 100% de los pacientes. Todos los pacientes mantuvieron una respuesta viral sostenida a las 12 semanas. Tres pacientes (33,3%) tuvieron algún tipo de efecto adverso (2 dolores de cabeza y un afta oral). La mediana de seguimiento posterior al tratamiento fue de 24 semanas (12-104).
ConclusionesEl tratamiento con ledipasvir/sofosbuvir en pacientes pediátricos con infección crónica por VHC de genotipo 1 y 4 es seguro y efectivo con RVS12, similar a lo reportado en adultos.
Infection by hepatitis C virus (HCV) is a global health problem, with up to 170 million individuals suffering from chronic infection worldwide and more than 350000 deaths a year due to related diseases.1,2 In Europe, the estimated prevalence of infection by HCV in the paediatric population is of 0.2–0.4%.2 Mother-to-child transmission is the main cause of infection in children.3,4 Between 4% and 7% of children born to mothers with monoinfection by HCV get infected by vertical transmission.5 The global rate of spontaneous clearance of HCV in vertically-infected children ranges from 24% to 30%.6 In patients in whom spontaneous clearance occur, it is usually before age 5 years.
Chronic HCV infection in children is usually asymptomatic or presents with mild and nonspecific symptoms and minimal abnormalities in liver function tests.7 Nevertheless, it produces a low-grade inflammation of the liver with a slowly progressing fibrosis, although cirrhosis and end-stage liver disease have also been described in these patients.7,8 There are also reports in the literature of patients with chronic HCV infection that have developed hepatocellular carcinoma or required a liver transplant during childhood or adolescence.9,10
In adult patients, the use of direct-acting antivirals (DAAs) has changed the treatment paradigm of infection by HCV, turning it into a curable disease with the use of well-tolerated oral treatments and a high rate of sustained virologic response (SVR), halting the progression of liver disease in many cases.11,12 These improvements in the treatment of adult patients are slowly being implemented in the paediatric age group. Until recently, paediatric patients have mainly been treated with 24–48-week regimens of pegylated interferon (PEG-INF) and ribavirin (RBV), which can have severe adverse effects.13 Recently, data have been published on the combined use of ledipasvir (LDV) and sofosbuvir (SOF) in children aged more than 6 years infected by HCV, showing that treatment with LDV/SOF at a dose of 45mg/200mg achieves outcomes similar to those observed in adults.14 There is also recent data on the safety and efficacy of combination therapy with LDV/SOF in adolescents infected with HCV genotype 1 infection that shows an overall SVR at 12 weeks post treatment (SVR12) of 98%.15 The combination of ribavirin and sofosbuvir has also proven highly effective in paediatric patients aged more than 12 years with HCV genotype 2 or 3, with up to 98% exhibiting a SVR at 12 or 24 weeks post treatment (depending on the genotype).16 For the above reasons, in 2017 the United States Food and Drug Administration (FDA) authorised the use of these 2 combinations (SOF/RBV and LDV/SOF) in patients aged more than 12 years.17,18
The aim of our study was to describe our clinical experience with the combination of LDV/SOF for treatment of paediatric patients with chronic infection by HCV genotype 1 and genotype 4.
Patients and methodsWe conducted a prospective, observational open-label single-centre study to assess the efficacy and safety of combined treatment with LDV/SOF in paediatric patients with chronic infection by HCV genotype 1 or genotype 4. The study protocol was reviewed and approved by the ethics board of our hospital, and we conducted it in adherence with the principles of the Declaration of Helsinki. At the time that we initiated treatment in our patients, LDV/SOF was authorised for use in adults but not in children. We obtained informed consent for every patient included in the study.
Inclusion criteriaThe patients eligible for treatment with LDV/SOF were patients aged 6–18 years with chronic infection by HCV genotype 1 or 4 and a viral load of more than 1000IU/mL. Before initiating treatment, candidates for treatment had to have a haemoglobin concentration of more than 11mg/dL and an absolute neutrophil count of more than 1500cells/mm3. Patients were included regardless of the presence of fibrosis, but patients with decompensated cirrhosis were excluded. We staged liver fibrosis by means of elastography (FibroScan®, Echosens; Paris, France) and/or examination of a liver biopsy specimen when available. We classified patients into 2 groups based on whether they had received previous treatment with PEG-INF+RBV.
We excluded patients with coinfection by hepatitis B virus, hepatitis A virus or human immunodeficiency virus or with a history of psychiatric disorders. We obtained the written informed consent of the parents or legal guardians of participants before starting the study protocol. We also obtained the written consent of patients aged more than 12 years.
TreatmentBefore starting treatment, all patients met with a paediatric hepatologist and a clinical pharmacologist to assess the risk of drug interactions. Treatment was implemented following the recommendations for treatment with DAAs of the American Association for the Study of Liver Diseases (AASLD).19 We treated patients aged 12–18 following the following recommendations: daily fixed-dose combination of ledipasvir (90mg)/sofosbuvir (400mg) for 12 weeks for treatment-naïve patients with HCV genotypes 1a, 1b or 4, with or without cirrhosis; daily fixed-dose combination of ledipasvir (90mg)/sofosbuvir (400mg) for 12 weeks for patients previously treated with PEG-INF+RBV with HCV genotypes 1a, 1b or 4 without cirrhosis; and daily fixed-dose combination of ledipasvir (90mg)/sofosbuvir (400mg) for 24 weeks for patients previously treated with PEG-INF+RBV infected with HCV genotype 1a, 1b or 4 with compensated cirrhosis.
For patients aged 6–12 years, we applied the following recommendations: daily fixed-dose combination of ledipasvir (45mg)/sofosbuvir (200mg) for 12 weeks for treatment-naïve patients infected with HCV genotype 1a, 1b or 4 without cirrhosis, and daily fixed-dose combination of ledipasvir (45mg)/sofosbuvir (200mg) for 24 weeks for patients previously treated with PEG-INF+RBV with compensated cirrhosis.
Primary endpointThe main variable used to assess efficacy was the presence of a sustained virologic response, which we defined as an HCV RNA level below the lower limit of quantitation at 12 weeks after completion of treatment.
Assessment of efficacyWe measured the serum viral load in each patient during the initial screening and at weeks 2, 4, 6, 8 and 12 weeks of treatment. In patients that received 24-week courses of pharmacotherapy, we collected additional blood samples to measure serum levels of HCV RNA at 16, 20 and 24 weeks. After completing treatment, we collected blood samples at 4, 8, 12 weeks and every 12 weeks thereafter. We measured serum HCV RNA levels with the AmpliPrep/COBAS TaqMan HCV test version 2.0 (Roche Molecular Systems, Inc; Branchburg, NJ, USA), which has a lower limit of quantitation of 15IU/mL.
Assessment of safetyDuring treatment, we collected data on patient vital signs, findings of the full physical examination, adverse events, concomitant pharmacotherapy and laboratory tests performed in each visit.
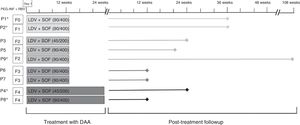
ResultsBetween March 2016 and July 2017, a total of 9 patients were treated with a combination of LDV/SOF in our hospital; 7 were male, and the median age of the cohort was 14.8 years (8.48–17.91). Table 1 presents the demographic characteristics of the patients. Six of the patients (66.6%) had acquired the infection by vertical transmission. One other patient had been adopted at age 3 years. While it is very likely that this patient was also infected by vertical transmission, no medical records were available that predated the adoption. Another patient had Fanconi anaemia and had acquired the infection through a bone marrow transplant from his father, who had HCV infection (and was the only available haploidentical donor). The remaining patient had received a kidney transplant and became infected during a dialysis session. Five patients with a median age of 8.2 years (3–15 years) had been treated previously with PEG-INF+RBV for a median of 8.5 months (3–12 months). Two of them had not responded to treatment, 2 had shown a partial response and 1 had experienced virologic relapse 3 months after completion of treatment. All patients had experienced side effects from treatment with PEG-INF+RBV (bone marrow failure in 2, fever and headache in 2, and lesion at the puncture site in 1). The 9 patients treated with LDV/SOF had an IL-28B polymorphism other than the CC genotype: 4 patients had genotype 1a, 4 patients genotype 1b and 1 patient genotype 4. At the time of treatment initiation, 8 of the 9 patients treated with LDV/SOF (88.8%) had some degree of fibrosis (stage F1 in 1 patient, F2 in 3 patients, F3 in 2 patients and F4 in 2 patients, one of them with regenerating nodules confirmed by biopsy examination). All patients attended the follow-up visits scheduled during treatment. Treatment lasted 12 weeks in 7 of the 9 patients and 24 weeks in 2 patients with cirrhosis that had been previously treated with PEG-INF+RBV. The median duration of followup after treatment was 24 weeks (12–104) (Fig. 1).
Demographic and virologic characteristics.
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | |
|---|---|---|---|---|---|---|---|---|---|
| Age at treatment initiation (years) | 14.85 | 17.34 | 8.48 | 9.11 | 12.31 | 17.91 | 12.08 | 15.48 | 17.31 |
| Sex | Male | Female | Male | Male | Male | Male | Female | Male | Male |
| Weight (kg) | 66.50 | 75.50 | 26.00 | 21.00 | 54.00 | 85.00 | 40.00 | 53.00 | 75.00 |
| Race | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | African-American | Caucasian | Caucasian |
| Genotype | 1b | 1b | 1b | 4 | 1b | 1a | 1a | 1a | 1a |
| IL28B | CT | CT | TT | CT | CT | TT | CT | CT | CT |
| Baseline HCV RNA (log10IU/mL) | 6.20 | 6.23 | 6.01 | 6.20 | 6.80 | 6.10 | 5.91 | 6.50 | 6.62 |
| HCV clearance (weeks) | 4 | 4 | 4 | 6 | 2 | 4 | 4 | 6 | 4 |
| Stage of fibrosis | F0 | F1 | F2 | F4 | F2 | F3 | F3 | F4 | F2 |
| Method used to assess fibrosis | Biopsy | Biopsy | Elastography | Biopsy | Elastography | Elastography | Elastography | Elastography | Elastography |
| Previous treatment with PEG-INF+RBV | Yes | Yes | No | Yes | Yes | No | No | Yes | No |
| Comorbidities | No | No | Fontan surgery | No | No | Bone marrow transplant (Fanconi anaemia) | Kidney transplant (lupus) | No | No |
| Baseline AST | 45.00 | 42.00 | 81.00 | 206.00 | 60.00 | 108.00 | 99.00 | 62.00 | 85.00 |
| Baseline ALT | 21.00 | 27.00 | 37.00 | 52.00 | 31.00 | 35.00 | 32.00 | 25.00 | 35.00 |
| Route of transmission | Vertical | Vertical | Unknown | Vertical | Vertical | Transfusion | Contaminated needle | Vertical | Vertical |
Treatment with direct-acting antivirals. The first column refers to the patient number. Patients previously treated with interferon-ribavirin are marked with an asterisk (*). The second column presents the stage of fibrosis before treatment based on the METAVIR score. The third column shows the dose and duration of treatment. The lines represent the duration of followup after completion of treatment.
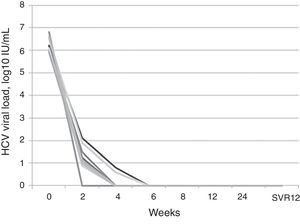
The median viral load before treatment was 6.2log10 (5.9–6.8), and HCV RNA became undetectable at 4 weeks of treatment in 77.7% (7/9) of the patients. The other 2 patients (22.2%) had an undetectable viral load at 6 weeks of treatment. All patients exhibited SVR 12 weeks after completion of treatment, independently of IL28B polymorphism, previous PEG-INF+RBV treatment and stage of fibrosis before treatment (Fig. 2).
SafetyThree patients (33.3%) experienced adverse events: mild headache in 2 (22.2%) and recurrent mouth ulcers in 1 (11.1%). None of the patients developed abnormalities in the complete blood count or the liver function tests during treatment. None of the patients experienced severe adverse events requiring discontinuation of treatment.
DiscussionHepatitis C virus is associated with a substantial burden of disease worldwide. Although infection by HCV in children in developed countries is nearly limited to mother-to-child transmission,4 in other parts of the world parenteral transmission continues to be a significant problem that causes high rates of infection by HCV.20 Until only a few months ago, the standard of care for children with chronic infection by HCV was a 24–48-week course of subcutaneous PEG-INF combined with oral RBV.13 Although this treatment was effective in some cases, it was associated with significant side effects.21 Advances in our understanding of the molecular structure and life cycle of HCV have allowed the development of new drugs widely known as direct-acting antivirals.3 Since 2013, DAA regimens have been approved for treatment of adults with chronic infection by HCV, with an excellent efficacy (SVR12>90%) and safety profile.8 The introduction of DAAs in the treatment of adult patients has completely shifted the paradigm of chronic HCV infection, turning it into a disease with a substantial cure fraction achieved with oral treatment and no significant adverse effects.11,12,22
Unfortunately, in children with chronic infection in Spain, DAA regimens are only available as off-label treatment, whereas in other countries they have already been approved for use in the paediatric population.17,18
There are some clear problems in the treatment of HCV in children. First of all, there is a dearth of studies on the pharmacokinetics, efficacy and safety of drugs in the paediatric population. Furthermore, while there are well-established guidelines for the treatment of HCV in adults, there is no consensus regarding which patients with HCV should be treated or the right timing for it. Also, the cost of treatment with DAAs, which is substantial even in developed countries, cannot be overlooked. However, given the outcomes reported by the most recent studies in the paediatric age group,15,16 which have demonstrated the safety and effectiveness of DAAs in this population, this approach should soon become the standard of care for children with HCV in Spain. Considering the excellent results achieved with DAAs in adult patients and the results of pilot studies conducted in paediatric patients, there seems to be no justification for the continued use of PEG-INF+RVB in children.
In our study, 9 paediatric patients with chronic infection by HCV genotype 1 or 4 received treatment with LDV/SOF for 12 or 24 weeks depending on previous treatment with PEG-INF+RBV and the stage of fibrosis. The proportion that achieved SVR at 12 weeks post treatment was 100%. This high efficacy of treatment was also associated with a rapid clearance of HCV, with RNA levels becoming undetectable by week 4 in 77% of patients and by week 6 in 100%. Some authors have reported that in treatment-naïve patients with mild fibrosis and low viral loads (<6log10IU/mL), 8-week courses of LDV/SVOF may achieve rates of SVR similar to those observed with 12-week courses.23 We ought to underscore that in our cohort, patients with compensated cirrhosis previously treated with PEG-INF+RBV were the patients whose viral loads became undetectable latest (in week 6), which supports the notion that shorter courses could be evaluated for the treatment of select patients in the future.
There are 2 main limitations to our study: (1) its small sample size, although despite the limited number of patients, it is reasonable to assume that DAA regimens could be at least as effective in children as they are in the adult population; and (2) the short duration of followup, as it would be important to evaluate outcomes in patients with more severe fibrosis over longer periods following viral clearance. As was the case in published studies conducted in adults, we found no differences in efficacy or in the development of adverse events based on genotype (1 or 4), IL28B polymorphism or previous treatment with PEG-INF+RBV. Although we documented the IL28B genotype in our study, none of the evidence in the literature has shown an association between IL28B polymorphism and the response to DAA therapy, contrary to the response to IFN-RBV therapy. For this reason, we do not consider IL28B genotype testing necessary in paediatric patients prior to initiating treatment with DAAs.
We also ought to highlight that we are presenting a real-world study that included one kidney transplant recipient, one bone marrow transplant recipient and one patient that had undergone heart surgery. We monitored patients closely, especially when it came to the levels of tacrolimus in the kidney transplant recipient. There was previous published evidence that treatment with LDV/SOF was safe in patients with end-stage renal disease or kidney transplant recipients with HCV infection.24 In our study, we found no changes in the levels of tacrolimus or renal function in the patient that had received a kidney transplant, who exhibited viral clearance by week 4.
As for the observed adverse events, they were all mild and very similar to those reported in studies in adults.12 None of the patients had to stop treatment due to side effects and none experienced severe or potentially fatal adverse events related to the medication under study.
To conclude, treatment with LDV/SOF in paediatric patients with chronic infection by HCV genotype 1 or 4 is safe and effective, with evidence of SVR at 12 weeks post treatment, similar to the outcomes of studies in adult patients. The dose use in adults seems to be well tolerated and effective in the treatment of adolescents. However, further studies in larger samples are required to generalise the use of DAA regimens to the paediatric population after confirming that it is cost-effective in curing the disease and preventing complications from an early stage.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Quintero J, Juampérez J, Julio E, Cabello V, Mercadal-Hally M, Soler-Palacín P, et al. Combinación de ledipasvir/sofosbuvir como tratamiento de la infección crónica por hepatitis C. An Pediatr (Barc). 2019;90:141–147.
Previous presentation: This study was presented as an oral communication at the XXIV Congress of the Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica; May 25–27, 2017; San Sebastian, Spain.