Kidney injury associated with paediatric liver transplantation (LT) is common, but its evaluation is challenging. Our aim was to analyse the presence of perioperative acute kidney injury (AKI) and study the prevalence of chronic kidney disease (CKD) using different glomerular filtration rate (GFR) estimation formulas.
MethodsWe conducted a cross-sectional study in a cohort of children aged less than 18 years with a history of LT followed up for 5.42 years. We estimated the GFR using the creatinine-based Schwartz bedside formula (2009), the cystatin C-based Caucasian Asian Pediatric and Adult cohort (CAPA) equation and the combined Full-Age Spectrum (FAS) formula as modified by Pottel. We analysed the agreement between them using the Bland–Altman method and the kappa statistic. We measured the albumin level in urine, the urine volume adjusted to 100 mL of GFR and blood pressure. We performed univariate and multivariate analyses of the risk factors associated with CKD.
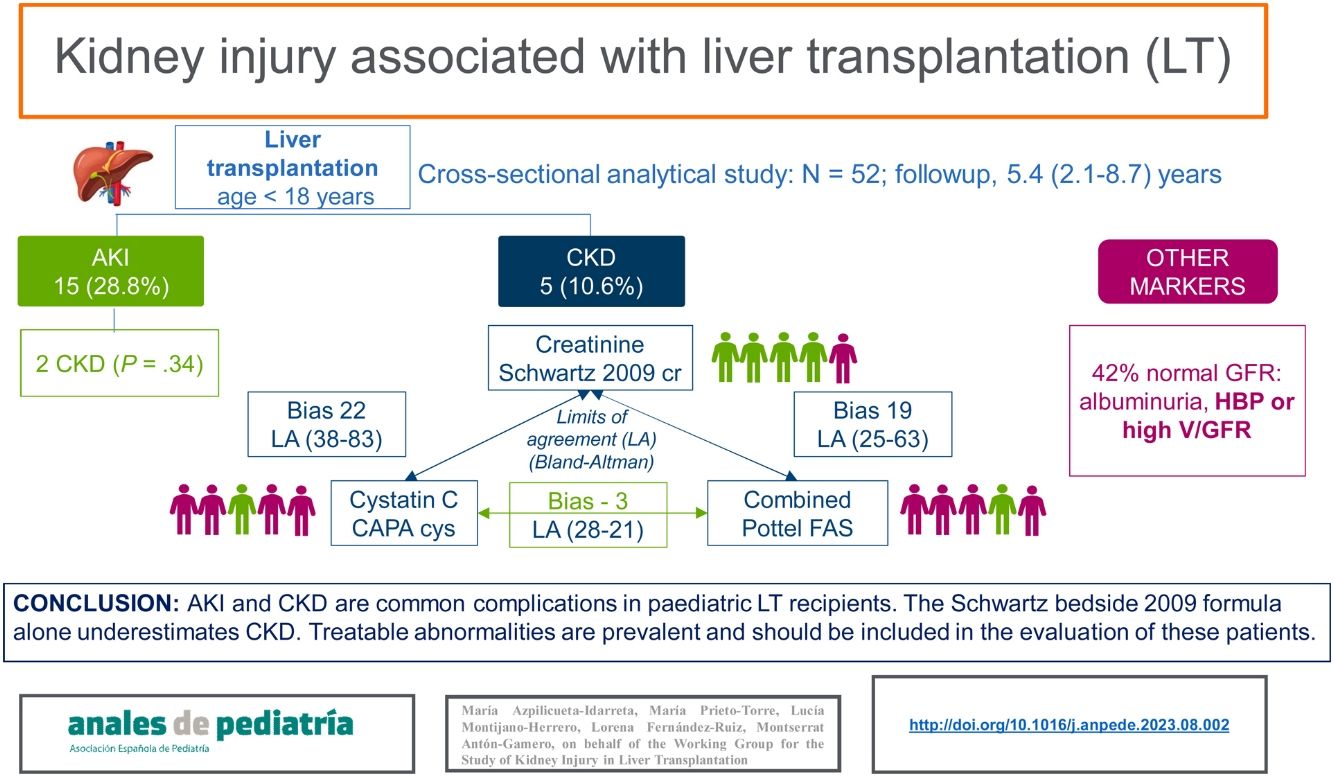
ResultsThe sample included 52 patients with a median age of 9.21 years. Fifteen (28.8%) had AKI. Five (10%) had CKD and the only associated risk factor was acute liver failure at the time of LT (odds ratio, 8.57; P = 0.04). There was poor agreement between the different estimation formulas. One patient was classified as having CKD with the Schwartz formula compared to four patients with the CAPA and the Pottel combined FAS formulas. Up to 42% of children without CKD had some positive marker of kidney injury.
ConclusionsThe exclusive use of the 2009 Schwartz bedside formula to estimate GFR may lead to underdiagnosis of CKD in children post LT. Other markers of kidney injury are common, and their detection may help prevent the progression of CKD.
El daño renal es frecuente en niños con trasplante hepático (TH), aunque su detección es un desafío. Nuestro objetivo fue evaluar el daño renal agudo (DRA) perioperatorio y analizar la prevalencia de enfermedad renal crónica (ERC) mediante diferentes fórmulas de estimación de la tasa de filtración glomerular (TFG).
MétodosAnálisis transversal unicéntrico de una cohorte de niños menores de 18 años con TH. Estimamos la TFG utilizando la fórmula Schwartz bedside 2009 basada en la creatinina, Caucasian Asian Pediatric and Adult cohort (CAPA) para cistatina C y la fórmula combinada de Pottel Full Age Spectrum (FAS). Analizamos la concordancia mediante prueba de Bland Altman y el índice kappa. Medimos la albuminuria, la presión arterial y el volumen urinario por 100 ml de filtrado glomerular. Analizamos los factores de riesgo asociados a ERC mediante un análisis univariante y multivariante.
ResultadosSe incluyeron 52 pacientes, con una mediana de edad de 9,21 años y 5,42 años de evolución. Quince (28,8%) tuvieron DRA. Cinco niños (10%) presentaban ERC. El único factor de riesgo asociado fue el fallo hepático agudo en el momento del TH (OR: 8,57, p = 0,04). Hubo poca concordancia entre las diferentes fórmulas de estimación. La fórmula de Schwartz clasificó a un paciente con ERC, mientras que Pottel FAS combinada y CAPA clasificaron a cuatro. Hasta el 42% de los niños sin ERC tenían algún marcador de daño renal.
ConclusionesEl uso exclusivo de la fórmula Schwartz bedside 2009 para estimar el FG puede limitar el diagnóstico de ERC en niños con TH. La presencia de otros marcadores de daño renal es frecuente y su detección puede prevenir la progresión de la ERC.
Liver transplantation (LT) is the treatment of choice for children with severe advanced liver disease.1 Improvements in surgical technique, postoperative care and immunosuppressive treatment have achieved an increase in survival in children with a LT to 95% at 1 year post transplantation and 85%–90% at 5 years.2 However, long-term complications may develop, such as chronic kidney disease (CKD), which is associated with an increase in morbidity and mortality,3 a decrease in liver graft survival and greater health care costs.3,4
The main causes of kidney injury in patients with LT are pre-existing kidney disease, perioperative acute kidney injury (AKI) and long-term nephrotoxicity of calcineurin inhibitors. 3,5 Its prevalence varies based on the characteristics of the samples under study, the duration of disease or follow-up and variations in the criteria used to define kidney injury and the methods used to estimate the glomerular filtration rate (GFR). Thus, in paediatric LT recipients, the incidence of AKI in the immediate postoperative period ranges between 17% and 57%6,7 and the prevalence of CKD at 5 years post transplantation is 17.6%.8 In adults, kidney involvement both pre- and post-LT is even more frequent and occurs in more than half of transplant recipients at 10 years of follow-up, which may be related to the higher prevalence of comorbidities.4
The evaluation of renal function in these patients is complex and has certain limitations. The gold standard for the study of glomerular function is measurement of the GFR using exogenous markers, which requires invasive procedures that are reserved for research.9 In clinical practice, mathematical formulas are used to estimate the GFR based on the serum value of creatinine or cystatin C.3 Few studies have analysed the application of these formulas in the context of LT.10,11 Estimation formulas based on creatinine, such as the 2009 Schwartz bedside formula, overestimate GFR in patients with liver disease.4,12 Formulas based on cystatin C seem to offer a greater agreement with exogenous GFR measurement methods.10,13
There is also scarce evidence on the early markers of kidney injury in these patients, such as tubular renal water handling, albuminuria and high blood pressure (HBP).14,15
Despite these limitations, the assessment of kidney injury in paediatric LT recipients can help identify patients at higher risk and detect treatable risk factors in order to modify care delivery and prevent or reduce the progression of kidney disease.
The aim of our study was to analyse the prevalence and characteristics of perioperative AKI, CKD, and other parameters of kidney injury in a cohort of paediatric LT recipients. We also analysed the concordance of different GFR estimation formulas.
MethodsWe carried out a cross-sectional analysis of a cohort of children aged less than 18 years who had undergone LT and managed at the outpatient clinic of a tertiary care hospital between January 2019 and February 2020. We excluded patients with impaired liver function, who had undergone multiple organ transplantation or liver retransplantation or with a post-transplantation follow-up duration of less than 6 months.
We calculated the prevalence of perioperative AKI according to the 2012 KDIGO criteria by performing a retrospective review of the health records,16 classifying cases based on the creatinine criteria alone.
At the time of the visit, we recorded anthropometric measurements obtained with standardized methods, calculated the body mass index (BMI), and classified patients by nutritional status for age and sex.17,18 We collected venous blood samples for measurement of serum creatinine with the colorimetric method with IDMS traceability and serum cystatin C by the turbidimetric method, and a first morning urine sample for measurement of creatinine and albumin levels. We also recorded the findings of the most recent ultrasound examination.
We estimated the GFR using the 2009 creatinine-based Schwartz bedside formula for creatinine (2009 Schwartz cr), the Caucasian Asian Pediatric and Adult Cohorts (CAPA) for cystatin C (CAPA cys) and the Full Age Spectrum (FAS) combined with creatinine as modified by Pottle (Pottel cr-cys FAS)10,13 (Table 1). We defined two groups based on the presence or absence of CKD based on any of the formulas used according to the KDIGO criteria.19
Glomerular filtration rate estimation formulas (mL/min/1.73 m2).
| Creatinine:Schwartz bedside 2009 | k × height (cm)/sCr• ≥12 months, k = 0.413 |
| Cystatin C:CAPA cys 2014 | 130 × (cystatin C−1.069) × age–0.117 − 7 |
| Combined FAS 2017 (Pottel) | 107.3/[((0.5 × sCr/(0.027 × age in years + 0.2329)) + (0.5 × cystatin C/0.82)] |
CAPA, Caucasian Asian Pediatric Adult Cohort; cys, cystatin C; FAS, Full-Age Spectrum; sCr, serum creatinine.
The urine volume adjusted to 100 mL of GFR (V/GFR) was calculated with the following formula: serum creatinine × 100/urine creatinine, and renal water handling was considered to be impaired when the value was greater than 1.03 mL/100 mL of GFR. We also calculated the urine osmolality, considering values inferior to 800 mOsm/L abnormal.20,21 We analysed the albumin/creatinine ratio expressed in mg/g,19 measured the casual blood pressure with the oscillometric method and assessed for the presence of HBP.22
We collected data on patient-related variables such as age, sex, race, liver disease (identifying those with potential renal involvement) and the pre-transplantation end-stage liver disease score (Pediatric End-Stage Liver Disease [PELD] for patients under 12 years, and Model For End Stage Liver Disease [MELD] for older patients) at the time of inclusion in the LT programme. We also collected data on perioperative variables: type of donor, acute rejection, cardiopulmonary and graft complications, sepsis, length of stay in the paediatric intensive care unit (PICU) in days, and presence of AKI.
The patients received immunosuppressive treatment according to the current protocol at the time of transplantation. We defined tacrolimus intoxication as a through blood level greater than 15 ng/mL in the post-LT period.1
Qualitative variables are expressed as numbers and proportions, and quantitative variables as the mean and standard deviation or median and interquartile range, depending on the distribution of the data. We used the Shapiro–Wilk test was employed to assess the normality of the distribution and the Levene test to assess the homogeneity of variance. We compared proportions with the χ2 test. Continuous variables were compared between patients with and without AKI and CKD with the Student t test for unpaired data and the Mann–Whitney U test based on whether or not the data followed a normal distribution.
We studied the risk factors for CKD by means of a logistic regression model, calculating odds ratios (ORs) and 95% confidence intervals (CIs). The variables that showed an association with a P value of less than 0.25 were included in the multiple logistic regression analysis. Using the backward stepwise method, we eliminated variables with Wald statistic values of 0.15 or greater from the model one by one until we obtained the estimate for the adjusted OR. Race was excluded from the multivariate study because we detected a statistical interaction.
The 3 GFR estimation equations were compared with analysis of variance (ANOVA) with the Sidak correction for post hoc comparisons. We studied the correlation between the GFR values obtained with the different formulas using the Spearman rho (ρ) and the agreement using the Bland–Altman method. We calculated the kappa coefficient (κ) to assess the agreement in the percentage of patients classified as having CKD using the different GFR estimation formulas.
All tests were performed for two tails, and we considered a P value of less than 0.05 statistically significant. The statistical analysis was carried out with the SPSS package, version 24.0.
ResultsA total of 61 paediatric LT recipients were evaluated. We excluded 8 because they had received more than one graft and 1 because they had undergone LT in the past 6 months. The final sample comprised 52 patients (25 male) with a median age of 9.21 years and a duration of follow-up post LT of 5.42 years. Table 2 presents the main demographic and clinical characteristics of the sample. The most frequent causes of LT were congenital anomalies of biliary tract (59.6%), chiefly biliary tract atresia (46.15%). Other aetiologies included acute liver failure (17.3%), metabolic diseases (11.53%), hepatocellular cirrhosis (9.61%), and tumours (1.92%). Five patients had potential renal involvement, 3 had a diagnosis of Alagille syndrome and 2 a diagnosis of Caroli disease, although none had CKD before LT. Patients with metabolic diseases in our series did not have renal disease (Table 3). A single patient had right renal hypoplasia, identified in the ultrasound examination.
Demographic, anthropometric, and clinical characteristics.
| All patients (n = 52) | |
|---|---|
| Sex: male/female | 25 (48%)/27 (52%) |
| Race | |
| Caucasian | 39 (75%) |
| Latin American | 2 (3.8%) |
| Arab | 11 (21.2%) |
| Age at the time of visit (years) | 9.21 (3.9) |
| Age at the time of LT (years) | 1.5 (1.62) |
| BMI (kg/m2) | |
| Overweight or obesity | 15 (29%) |
| Normal | 32 (61%) |
| Undernutrition | 5 (10%) |
| Duration of follow-up (years) | 5.42 (3.29) |
| Disease with potential renal involvement | 5 (9.61%) |
| Acute liver failure | 9 (17.3%) |
| PELD/MELD | 16.5 (12–36.2) |
| Living donor | 15 (28.8%) |
| PICU stay (days) | 8.5 (10) |
| Perioperative AKI | 15 (28.8%) |
| Acute graft rejection | 10 (19.2%) |
| Graft complications | 22 (42.3%) |
| Cardiopulmonary complications | 21 (40.4%) |
| Sepsis | 7 (13.5%) |
| Treatment with calcineurin inhibitors | 38 (73.1%) |
| ≥2 tacrolimus intoxications | 22 (42.3%) |
| HBP | 22 (42.3%) |
| Creatinine (mg/dL) | 0.44 (0.83) |
| Cystatin (mg/L) | 0.87 (1.1) |
Quantitative variables are expressed as median (interquartile range) and qualitative variables as n (%).
AKI, acute kidney injury; BMI, body mass index; HBP, high blood pressure; LT, liver transplant; MELD, Model for End-stage Liver Disease score; PELD, Pediatric End-stage Liver Disease score; PICU, paediatric intensive care unit.
Diseases leading to liver transplantation.
| Anomaly of the biliary tract (59.6%) | Biliary tract atresia (46.15%) |
| Alagille syndromea (5.76%) | |
| Caroli diseasea (3.84%) | |
| Idiopathic (3.84%) | |
| Acute liver failure (17.3%) | Idiopathic (9.62%) |
| Hepatitis A (7.69%) | |
| Metabolic disease (11.53%) | Ornithine transcarbamylase deficiency (5.76%) |
| Citrullinaemia (3.84%) | |
| Glycogen storage disease type III (1.92%) | |
| Hepatocellular cirrhosis (9.61%) | Idiopathic (7.69%) |
| Autoimmune (1.92%) | |
| Tumour (1.92%) | Hepatoblastoma (1.92%) |
Values expressed as percentages.
Fifteen children (28.8%) had perioperative AKI. It only progressed to CKD in 2 patients (Table 4), both of who had acute liver failure.
Classification of acute kidney injury applying KDIGO 2012 serum creatinine criteria.
| Stage | n |
|---|---|
| 1. 1.5–1.9 times baseline or ≥0.3 mg/dL increase | 2 (1 with CKD) |
| 2. 2–2.9 times baseline | 7 |
| 3. 3 times baseline or ≥4 mg/dL increase or initiation of renal replacement therapy or in patients <18 years, decrease in eGFR to <35 mL/min per 1.73 m2 | 6 (1 with CKD) |
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
In the short term, there were no significant differences except for a higher incidence of sepsis (33.3% vs 5.6%; P 0.01). The prevalence of albuminuria or of HBP in the long term was not significantly different in patients who had perioperative AKI. Patients who required continuous renal replacement therapy (CRRT) had a significantly higher V/GFR (1.44 [0.95] vs 0.78 [0.44]; P 0.02).
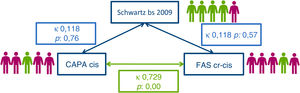
Chronic kidney diseaseFive patients (10.6%) were classified as having CKD based on at least one of the GFR estimation formulas, none of whom received CRRT. In this subset of patients, the indication for LT was biliary tract atresia in 2 and acute liver failure in the other 3. Only 1 patient was classified as having CKD (stage 2) based on the 2009 Schwartz cr formula. The CAPA cys and Pottel cr-cys FAS formulas led to the classification of 4 patients as having CKD (stage 2 in two and stage 3 in 2), although there was diagnostic disagreement in 2 patients (Fig. 1). We found no significant differences in the demographic or clinical characteristics of the patients with CKD versus the patients with a normal GFR, except for the frequency of Arab descent (60% vs 20%; P 0.01) and acute liver failure (60% vs 12.76%; P 0.03). In the univariate and multivariate analysis, the only risk factor associated with the presence of CKD was the history of acute liver failure (Table 5).
Classification of chronic kidney disease according to estimates obtained with different glomerular filtration rate formulas.
CAPA, Caucasian Asian Pediatric Adult cohort formula; cr, creatinine; cys, cystatin; FAS, Full-Age Spectrum formula; Schwartz bs 2009, Schwartz bedside 2009 formula; κ, kappa statistic.
Univariate and multivariate logistic regression analysis of the association of risk factors with CKD.
| Factors associated with CKD | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Sex | 1.423 | 0.22–9.4 | 0.705 | |||
| Age at time of transplantation | 0.93 | 0.707–1.24 | 0.665 | |||
| Duration of follow-up | 1 | 0.789–1.2 | 0.997 | |||
| Disease with PRI | 0.950 | 0.097–9.273 | 0.965 | |||
| Peri-LT AKI | 1.7 | 0.16–17.96 | 0.656 | |||
| PELD | 1.06 | 0.98–1.16 | 0.13 | |||
| ≥2 tacrolimus intoxications | 2.2 | 0.33–14.5 | 0.409 | |||
| ALT (U/L) | 1.013 | 0.99–1.029 | 0.103 | |||
| Acute liver failure | 8.57 | 1.2–60.9 | 0.03 | 8.50 | 1.2–60.9 | 0.032 |
ALT, alanine aminotransferase; AKI, acute kidney injury; CI, confidence interval; CKD, chronic kidney disease; LT Liver transplantation; OR, odds ratio; PELD, Pediatric End-stage Liver Disease score; PRI, potential renal involvement.
Sixty percent of patients with CKD and 42% of those who maintained a normal GFR had a positive marker of kidney injury. In patients with CKD, the prevalence of HBP was 27% and the prevalence of albuminuria 17% compared to 29% and 10%, respectively, in patients with a normal GFR. Only one patient received enalapril for treatment of HBP. A high V/GFR with abnormal urinary osmolality was detected in 28% of patients with CKD compared to 18% of patients with a normal GFR.
Concordance in the estimation of the GFRThe GFR estimated with the 2009 Schwartz cr formula was positively correlated to the estimates obtained with the cystatin C-based formula (CAPA cys: ρ, 0.553; P < 0.001) and the combined cystatin C-creatinine formula (Pottel cr-cys FAS: ρ, 0.789; P < 0.001). However, the mean values of the latter two were significantly higher (P < 0.001) (Table 5).
The level of agreement between the formulas, assessed with the Bland–Altman method, was not acceptable. The bias and the 95% limits of agreement were very broad in all cases, except when comparing the CAPA cys and Pottel cr-cys FAS formulas, in which the bias was −3.32 (Table 6 and Fig. 2).
Agreement between GFR estimation formulas. Bland–Altman analysis.
| GFR | GFR (mL/min/1.73 m2) | P (ANOVA) | Bland–Altman analysis | |||
|---|---|---|---|---|---|---|
| Bias (limits of agreement) | ||||||
| Mean ± SD | Range | CAPA cys | FAS cr-cys | CAPA cys | FAS cr-cys | |
| Schwartz bedside, 2009 | 129.33 ± 36.5 | 118.84–139.8 | 0.001 | 0.001 | 22.75 (−38.07 to 83.21) | 19.13 (−25.63 to 63.89) |
| CAPA cys | 106.57 ± 26 | 99.1–114 | 0.052 | −3.3 (−28.65 to 21.37) | ||
| FAS cr-cys | 110.1 ± 24.4 | 103.18–117.2 | ||||
CAPA, Caucasian Asian Pediatric Adult Cohort; cr, creatinine; cys, cystatin; FAS, Full-Age Spectrum; GFR, glomerular filtration rate; SD, standard deviation.
Bland–Altman plots for comparison of different estimation glomerular filtration rate formulas.
CAPA cys, cystatin-based Caucasian Asian Pediatric Adult cohort formula; cr, creatinine; cys, cystatin; Pottel FAS cr-cys, Full-Age Spectrum formula modified by Potter; Schwartz bs 2009; Schwartz bedside 2009 formula.
The red line represents bias and the green lines the limits of agreement. The values are presented in Table 6.
The 2009 Schwartz cr formula only classified 1 patient as having CKD, while the CAPA cys and Pottel cr-cys FAS formulas classified 4 of the 5 patients, showing poor agreement between them (Fig. 1).
DiscussionKidney injury is common in children with LT and is possibly underestimated due to its slow and silent progression.8 We present the first study in our country evaluating kidney damage in a series of 52 children from a single referral centre for LT. The demographic and aetiological characteristics were similar to those of previous studies1 except for the higher frequency of patients of Arab descent in our series with acute liver failure. Our unit is a referral LT centre for children from North African countries who receive a diagnosis of acute liver failure, which might be a source of bias in our study. Nearly 10% of our patients suffered from diseases with potential kidney injury, such as Alagille syndrome or Caroli syndrome. None of them had CKD pre or post transplantation.
Fifteen children (28.8%) had perioperative AKI, a slightly lower percentage than previously reported, of up to 57%,6,7,23 which may be due to the variability in the criteria used to diagnose AKI.23
Although AKI is a known factor for progression to CKD,23 only 2 of the children who had AKI in our series progressed to CKD. In the long term, we did not find a higher frequency of albuminuria or HBP (classic markers of kidney damage) in patients with AKI. A greater proportion of those who required CRRT had an abnormal V/GFR and urinary osmolality, although these findings would need a water deprivation test for the diagnosis of abnormal renal water handling.21 The ability of the kidney to concentrate urine is complex and requires normal tubular function. Loss of urinary concentration capacity is an early and simple marker to detect kidney injury20 that has not been previously reported in children with LT.
The prevalence of CKD in LT recipients is as high as 50% in adults and is mainly determined by the presence of comorbidities and calcineurin inhibitor nephrotoxicity.3 The prevalence is lower in children, of around 17% at 5 years, although it varies widely between published series.8 Despite the recommendation to use international criteria according to the KDIGO guidelines for the definition of CKD,19 different criteria are applied in the different studies, contributing to this variability. For instance, Yasui et al.24 defined renal dysfunction as a GFR of less than 90 mL/min/1.73 m2 without taking into account other associated factors, such as albuminuria or structural damage, and found that the estimated prevalence ranged from 3.8% to 15.3% in 26 paediatric living-donor LT recipients depending on the applied formula. Basiratnia et al.25 used the KDIGO criteria and reported a prevalence of stage 3 CKD from 2% to 33% in 46 paediatric LT recipients depending on the applied formula. In our cohort, applying the KDIGO classification, the prevalence of CKD was 10.6%, and it ranged from 1.9% with the 2009 Schwartz cr formula to 9.6% with the others. This variability was already described in a larger series by Brinkert et al., who found a prevalence ranging from 1.8% to 20% in 168 children with a LT depending on whether they used the 2009 Schwartz cr formula or the cystatin C-based Filler equation.26
Measuring the GFR is expensive and invasive, which limits its application in clinical practice. Equations have been developed to estimate the GFR with ease and precision. The most widely used equations are based on the measurement of serum creatinine, such as the 2009 Schwartz cr formula. However, their use has significant limitations in children with malnutrition, decreased muscle mass, and liver cirrhosis.12 Cystatin C is an endogenous marker that is independent of these factors. Estimation equations based on cystatin C are more sensitive to changes in the GFR and their use is preferred in cases in which serum creatinine is not a valid parameter.27 Studies that have analysed the agreement between formulas based on creatinine and cystatin C in paediatric LT recipients have obtained results similar to ours, confirming the overestimation of the GFR with the use of formulas based on creatinine without finding an acceptable agreement with cystatin C-based formulas.10 However, when compared with an exogenous measurement method, the CAPA cys formula exhibited good agreement, which suggests that cystatin C should be used at least once a year and more frequently if there is a decrease in GFR.10,13
We were unable to identify the previously described CKD risk factors, possibly due to the short duration of follow-up, the small sample size or the difficulty in assessing the impact of tacrolimus nephrotoxicity beyond counting the number of intoxications. The association of acute liver failure as the cause of LT with CKD has not been previously described. Perioperative AKI was more frequent in patients with acute liver failure and could explain this finding, although this difference was not statistically significant and would require confirmation in a larger sample.
We found children who, despite not suffering from CKD, had albuminuria, HBP or an abnormal V/GFR. These are markers of kidney injury whose long-term significance should be assessed, since they are related to the progression of renal dysfunction. These findings had not been previously reported in this population and may be relevant, as they would allow early identification and potential treatment of kidney injury.
LimitationsThe main limitations of our study are those intrinsic to its cross-sectional design, which did not allow verification of the stability of the findings over time. Although we classified CKD stages according to the KDIGO guidelines, due to the retrospective design of our study, a 3-month follow-up period is necessary to assure the CKD diagnostic criteria. Also, several measurements over time are necessary to confirm albuminuria and HBP. We did not use a direct GFR measurement method as the gold standard, so we could only reflect on the variability of the GFR estimation results based on the equation used and the relationship between them, and were unable to determine which is more reliable. Last of all, we could not assess AKI based on the urine output due to the difficulty of measuring this parameter.
ConclusionAcute and chronic kidney disease were frequent complications in the cohort of paediatric LT recipients under study (28.8% and 10.6%, respectively) after a substantial mean duration of liver disease in childhood. Up to 42% of children without CKD had some marker of kidney injury.
The evaluation of kidney injury in this population facilitates prevention, early diagnosis, classification and treatment of kidney disease and could improve long-term outcomes.16,19 However, there is no recommendation regarding which formulas to use for the estimation of the GFR, which exhibit a low agreement. The exclusive use of the 2009 Schwartz cr formula may result in underdiagnosis, and it is likely that the formulas based on cystatin C or combining creatinine and cystatin C or including demographic parameters are more accurate for estimation of the GFR. We recommend including other markers in the evaluation of kidney injury.
Conflict of interestThe authors have no confilct of interest.
María Azpilicueta-Idarreta: Unit of Paediatric Nephrology, Hospital Universitario Reina Sofía, Cordoba, Spain.
María Prieto-Torre: Department of Gastroenterology, Hospital Universitario Reina Sofía, Cordoba, Spain.
Lucía Montijano-Herrero: Department of Paediatrics, Hospital Universitario de Toledo, Toledo, Spain.
Lorena Fernández-Ruiz: Department of Paediatrics, Hospital Universitario Poniente, El Ejido, Almería, Spain.
María José Torre-Aguilar: Paediatric Research and Metabolism Unit, Instituto Maimónides de Investigación Biomédica de Córdoba (IMIBIC), Hospital Universitario Reina Sofía, Universidad de Córdoba, Cordoba, Spain.
Mónica Rodríguez-Salas: Unit of Paediatric Liver Transplantation, Hospital Universitario Reina Sofía, Cordoba, Spain.
Juan José Gilbert-Pérez: Unit of Paediatric Liver Transplantation, Hospital Universitario Reina Sofía, Cordoba, Spain.
Elena López-Vargas: Department of Paediatrics, Hospital Público Santa Bárbara, Puertollano, Ciudad Real, Spain.
Montserrat Antón-Gamero: Unit of Paediatric Nephrology, Hospital Universitario Reina Sofía, Universidad de Córdoba, Instituto Maimónides de Investigación Biomédica de Córdoba (IMIBIC), Cordoba, Spain.
Previous meeting: the results of this study were presented as an oral communication at the XLV Congress of the Asociación Española de Nefrología Pediátrica, May 2022, La Coruña, Spain.
Appendix Alists the members of the Working Group for the Study of Kidney Injury in Liver Transplantation.