Invasive meningococcal disease (IMD) has a high morbidity and mortality in children and adults. The aim of this study was to describe the clinical and epidemiological characteristics of patients with IMD, to compare them among children and adults, and to determine prognostic factors and changes in epidemiology during a 14-year period.
MethodsA retrospective study was conducted on patients admitted to a third level hospital with IMD between 2004 and 2017. An analysis was made of the clinical, epidemiological and microbiological data.
ResultsA total of 84 patients were diagnosed with IMD, of which 50 (59.5%) were children. Median age was 2 years (IQR 0.7–7.5) for children and 41.2 years (IQR 26.4–69.3) for adults. Diagnosis was bacteraemia in 47 patients (56%), meningitis in 24 (28.6%), and both in 13 (15.5%). Serogroup B (MenB) was the most common cause of IMD (40.5%), followed by serogroup C (MenC) in 15.5%, which was more common among adults (26.5% vs 8%, P=.022). Incidence rate decreased between 2004–2010 and 2011–2017, from 3.14 to 1.33cases/100000 emergencies attended in the study hospital (P<.001). Eighty-four percent of children had received ≥1 dose of vaccine against MenC, with none against MenB. Children had higher proportion of ICU admissions (78% vs 44.1%, P=.001). Mortality was slightly higher in adults (11.8% vs 2.0%, P=.153). Adverse outcomes (sequelae or mortality) were independently associated with intubation and thrombocytopenia, while disease severity with leukopenia and purpuric rash.
ConclusionsIMD incidence has decreased in our setting, with MenB being the most common serogroup. The higher prevalence of MenC in adults was probably related to lower vaccination coverage. According to this study, thrombocytopenia, leukopenia, and purpuric rash were parameters associated with worse outcome.
La enfermedad meningocócica invasiva (EMI) supone una causa importante de morbimortalidad en niños y adultos. Objetivo principal: describir las características clínicas y epidemiológicas de los pacientes con EMI. Objetivos secundarios: describir las diferencias entre niños y adultos, factores pronósticos y cambios epidemiológicos en los últimos 14 años.
MétodosEstudio retrospectivo realizado en un hospital terciario. Se incluyeron los pacientes diagnosticados de EMI entre 2004 y 2017, recogiéndose datos epidemiológicos, clínicos y microbiológicos.
ResultadosFueron diagnosticados 84 pacientes con EMI, 50 (59,5%) niños. Edad mediana en niños 2 años (RIC: 0,7-7,5) y adultos 41,2 años (RIC: 26,4-69,3). Bacteriemia en 47 casos (56%), meningitis en 24 (28,6%) y ambas en 13 (15,5%). Predominio del serogrupo B (MenB), en el 40,5%, seguido del serogrupo C (MenC), en el 15,5%, con mayor proporción de MenC en adultos (26,5 vs. 8%; p=0,022). Disminución en la incidencia de 2004-2010 a 2011-2017, pasando de 3,14 a 1,33 casos/100.000 urgencias en el centro de estudio (p<0,001). El 84% de los niños había recibido≥1 dosis de vacuna frente a MenC, ninguno frente a MenB. Mayor proporción de ingreso en UCI en niños (78 vs. 44,1%; p=0,001). Tendencia a mayor letalidad en adultos (11,8 vs. 2%; p=0,153). La intubación y la trombocitopenia fueron factores de riesgo independientes de desenlace adverso, y la leucopenia y el exantema purpúrico de gravedad.
ConclusionesSe objetivó un descenso en la incidencia de EMI, siendo MenB el mayoritario. El mayor porcentaje de MenC en adultos probablemente esté relacionado con una menor cobertura vacunal. La trombocitopenia, la leucopenia y el exantema purpúrico fueron factores de riesgo relacionados con peor pronóstico.
Invasive meningococcal disease (IMD), despite advances in its prevention and treatment, continues to be a serious health problem due to its high morbidity and mortality.1 The main preventive strategy that has achieved a reduction in mortality due to IMD is vaccination. In Spain, immunisation with the meningococcal group C (MenC) conjugate vaccine has resulted in a substantial decrease in the incidence of IMD,2 and group B meningococcus (MenB) is the most prevalent serogroup at present.2 The MenB and MenACWY vaccines have been recently included in the immunisation schedule for use in the general population, and are expected to further reduce the incidence of IMD in Spain.3,4
The primary objective of our study was to describe the clinical and epidemiological characteristics of patients admitted to a tertiary care hospital due to IMD, as well as the microbiological characteristics of isolates obtained in these patients. The secondary objectives were to assess the differences between the paediatric and the adult populations, identify predictors for poorer clinical outcomes and describe epidemiological trends in the past 14 years.
MethodsWe conducted a retrospective study in a tertiary care hospital in Madrid that has 120 paediatrics beds (age <17 years) and 1100 adult beds that manages an average of 60000 paediatric emergency visits and 160000 adult emergency visits a year.
We included patients with a final diagnosis of confirmed or probable IMD admitted to hospital between January 1, 2004 and December 31, 2017. We classified cases based on the definitions established by the Centers for Disease Control and Prevention5:
- -
Confirmed IMD: isolation of Neisseria meningitidis in culture or its detection by polymerase chain reaction (PCR) in a sample of fluid from a normally sterile site.
- -
Probable IMD: presence of compatible clinical picture (e.g. purpura fulminans) in the absence of microbiological confirmation.
We classified patients based on age as children (<17 years) or adults (≥17 years). We identified cases using the register of isolates of N. meningitidis of the Laboratory of Microbiology and the discharge summary database of the hospital, searching for patients with a discharge diagnosis of meningococcal infection documented with codes 036.0 to 036.9 of the International Classification of Diseases, Ninth Revision (ICD-9).6 For each case, we reviewed the health records to collect data on demographic variables, clinical presentation, laboratory results at admission, admission to the intensive care unit (ICU), microbiological results, length of stay and outcomes, including the presence of sequelae at discharge or identified during the outpatient followup.
We classified the clinical presentation as (a) meningitis, (b) bacteraemia/sepsis or (c) a combination of both. We defined meningitis as detection of N. meningitidis in cerebrospinal fluid (CSF) by culture or PCR or pleocytosis in CSF based on normal ranges applicable to the patient's age (>20 polymorphonuclear white blood cells [WBCs]/μL in patients <1 month and >5 polymorphonuclear WBCs/μL in patients >1 month). We defined bacteraemia/sepsis as isolation of N. meningitidis in blood culture or presence of purpura fulminans in a patient with compatible manifestations and sterile cultures. Last of all, we defined severe disease as need of invasive ventilatory and/or haemodynamic support, and adverse outcome as death or development of sequelae.
N.meningitidis isolates were identified by standardised techniques (biochemical tests and/or MALDI-TOF mass spectrometry) and typed by means of the kit BD Directigen Meningitidis Combo Test (BectonDickinson; Sparks, MD, USA) in the Microbiology Laboratory of our hospital. In addition, the laboratory subsequently submitted all isolated strains to the reference laboratory of the Centro Nacional de Microbiología (National Centre of Microbiology) to confirm the capsular serotype. The antimicrobial susceptibility was assessed by the broth microdilution method using commercially available Sensititre STRHAE2 plates (ThermoScientific, West Sussex, United Kingdom) in cation-adjusted Mueller-Hinton broth supplemented with 5% horse blood lysate. We applied the susceptibility cut-off points recommended by the European Committee on Antimicrobial Susceptibility Testing (EUCAST).7 Production of β-lactamase was assessed with nitrocefin disks (Cefinase, BectonDickinson; Sparks, MD, USA). Direct detection in CSF by PCR was performed using the RealCycler Universal kit (Progenie Molecular; Valencia, Spain).
Statistical analysisWe performed a descriptive analysis, summarising categorical variables as absolute frequencies and percentages and continuous variables as median and interquartile range (IQR). We compared categorical variables by means of the χ2 or Fisher test and continuous variables with the Mann–Whitney U test after determining by means of the Kolmogorov–Smirnov test that the data distributions were not normal. We assessed the association of clinical, laboratory and demographic variables with patient outcomes (disease severity and poor outcome) with univariate analyses. In the analysis of sequelae, we excluded patients that died. We fitted a multivariate logistic regression model with the variables included in the univariate analyses to predict the risk of severe disease and adverse outcome. We included in the model the variables corresponding to P-values of less than .10 in the univariate analysis or that we considered likely to have an effect based on biological principles and the previous literature.8 We included variables in the predictive model by a stepwise method, successively eliminating those with weaker associations (likelihood of outcome determined by applying a level of significance of 0.10 in the Wald test). We performed the statistical analysis with the software SPSS® version 22 (Chicago, USA). We defined statistical significance as a P-value of less than .05.
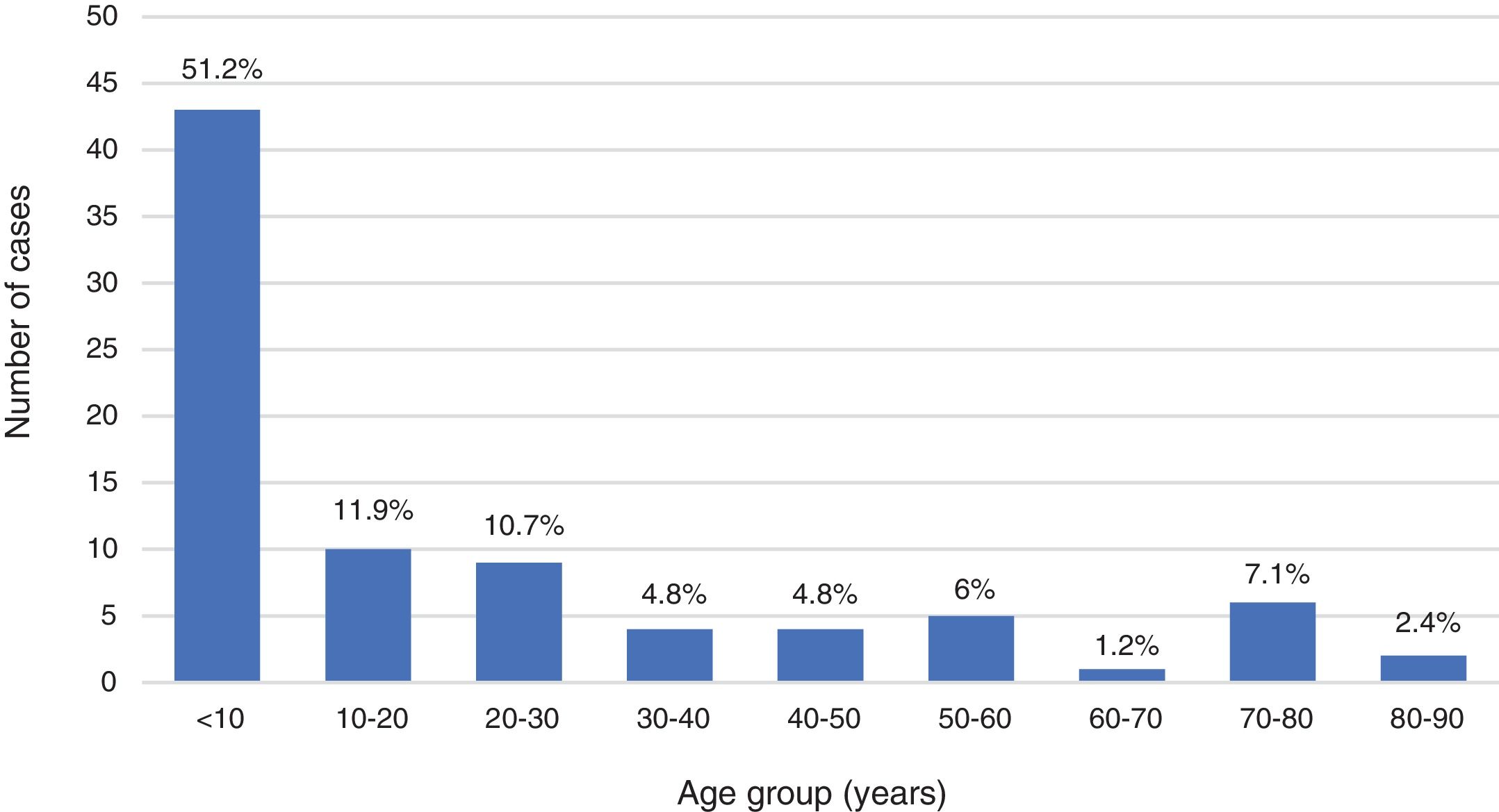
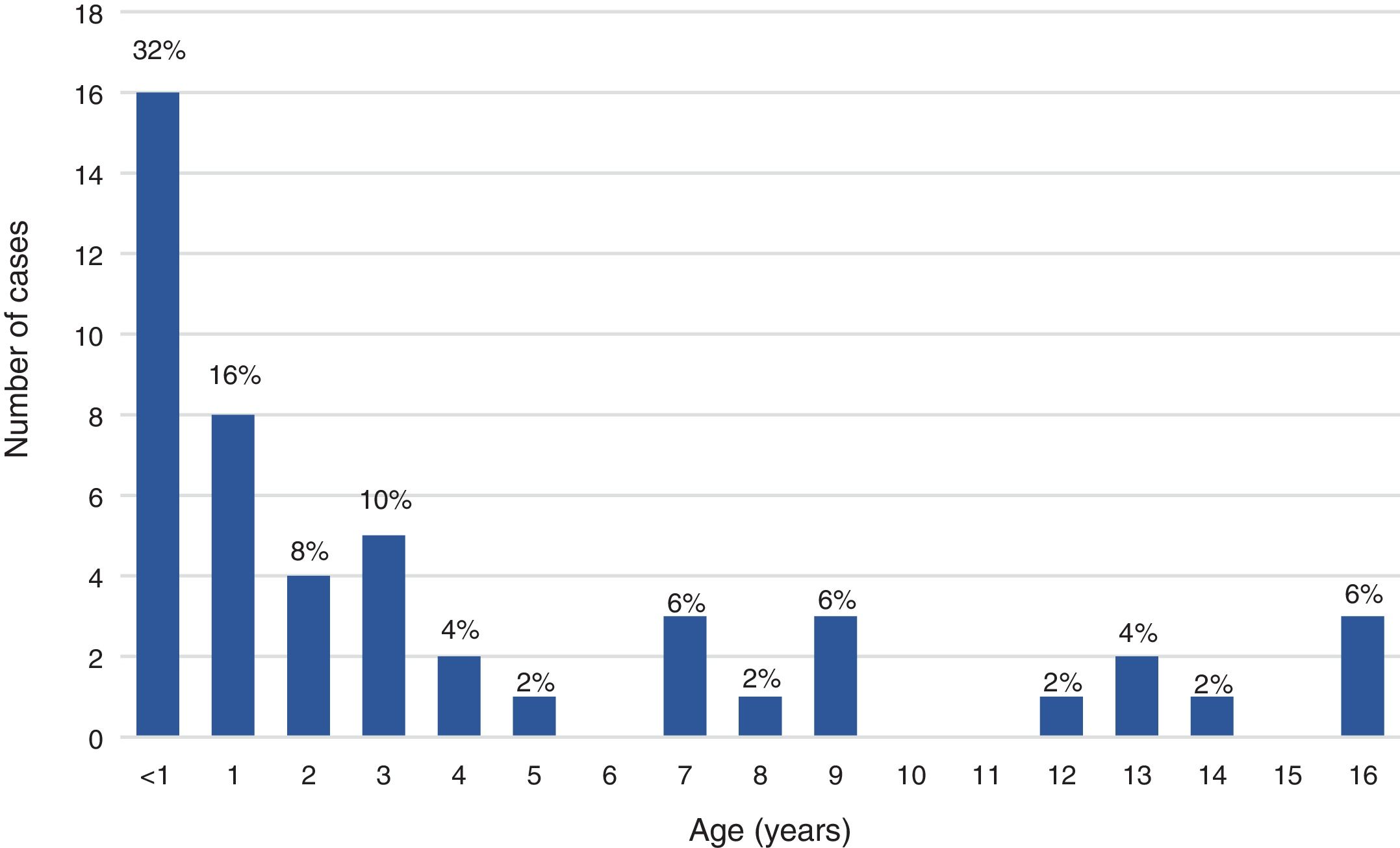
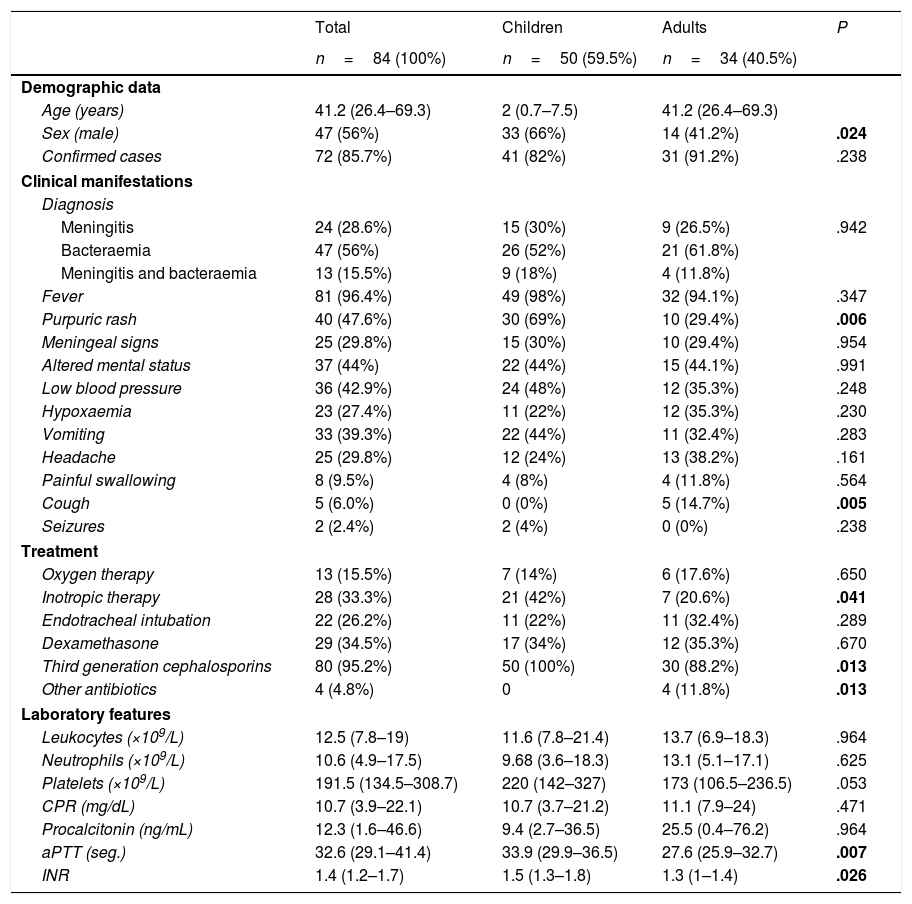
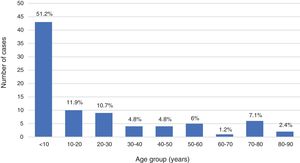
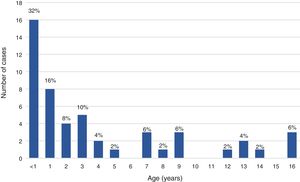
ResultsDuring the period under study, 84 patients received a final diagnosis of IMD, confirmed in 72 cases (85.7%) and probable in 12 (14.3%). Fifty patients (59.5%) were children and 34 (40.5%) adults, with an overall predominance of the male sex (56%), although with a higher proportion of female patients in the adult subset compared to the paediatric subset (58.8% vs 34%; P=.024). Thus, there was a predominance of boys in the paediatric subset (1.9:1) compared to a mild predominance of women in the adult subset (1:1.4). Of all cases of IMD, 28.6% occurred in children aged less than 2 years. The median age was 2 years in paediatric patients (IQR, 0.7–7.5) and 41.2 years in adult patients (IQR, 26.4–69.3). Figs. 1 and 2 show the age distribution of the total sample and the paediatric subset.
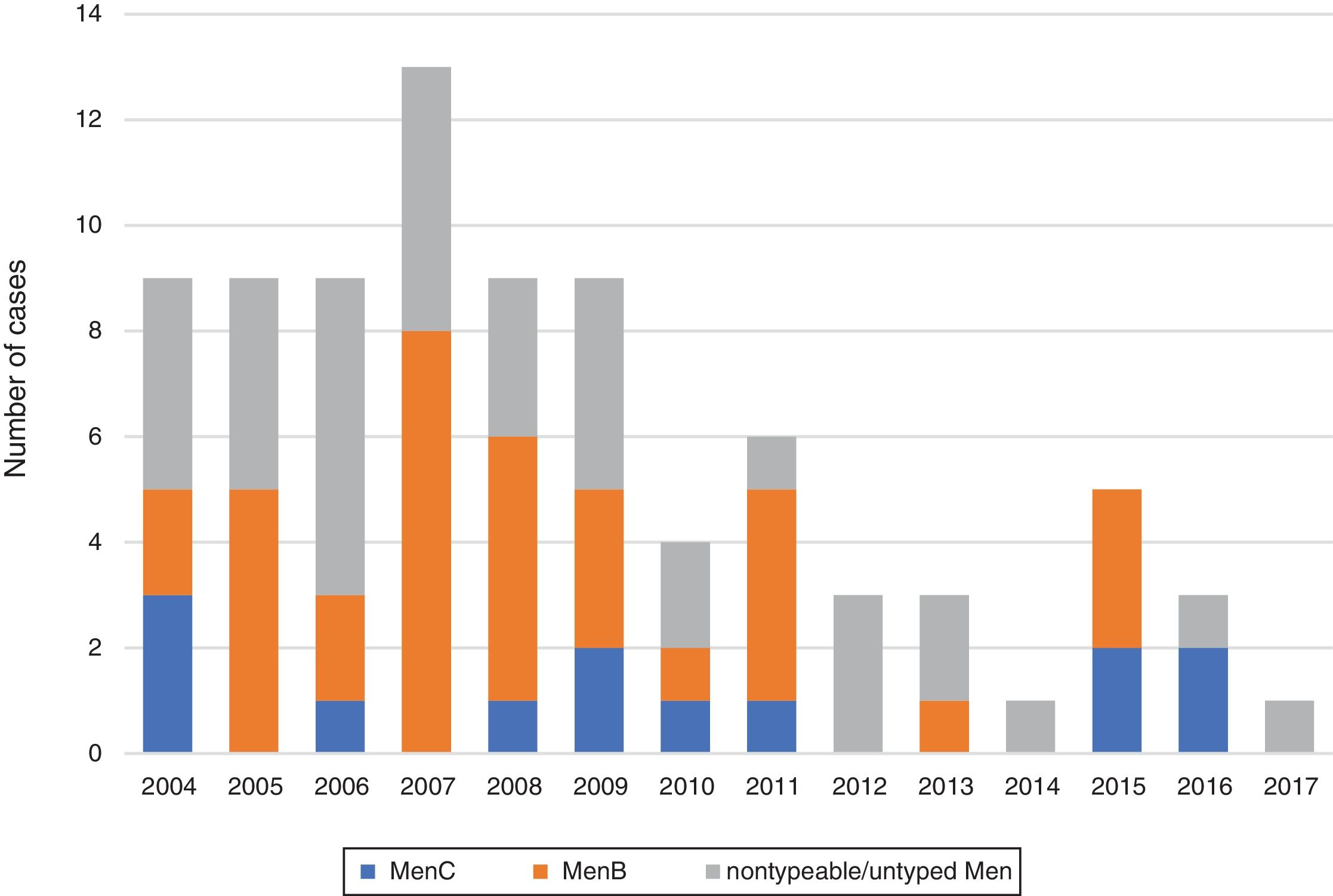
The mean annual incidence of IMD was 2.24 cases per 100000 visits managed in the emergency department of our hospital. However, we found a progressive decline in the annual absolute frequency of IMD (mean of 8.2cases/year in 2004–2010 vs 3.14 cases/year in 2011–2017) and its incidence when comparing the first period (2004–2010) with the second (2011–2017), as it decreased from 3.14 cases to 1.33 cases per 100000 visits managed in the emergency department (P<.001).
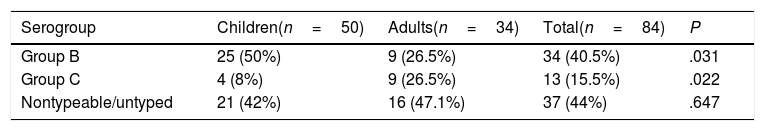
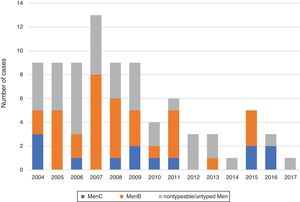
The most frequent serogroup was MenB (40.5%), followed by MenC (15.5%), with a higher proportion of MenC in the adult subset (26.5% vs 8%; P=.022). The serogroup was not identified in 44% of cases. Out of all confirmed cases, 69 (95.9%) were confirmed by culture, 2 (2.8%) by PCR (negative CSF cultures in both cases, with blood culture negative in 1 and not performed in the other) and 1 (1.4%) by both techniques. Table 1 shows the serogroup distribution by age group, and Fig. 3 the annual distribution of cases in the total sample by serogroup.
Of the 64 isolates that were available for antimicrobial susceptibility testing, 43 (67.2%) were fully susceptible to penicillin (minimum inhibitory concentration [MIC] ≤0.06mg/L). The MICs for penicillin in the remaining isolates ranged between 0.12 and 1mg/L. None of the isolates tested positive for β-lactamase production and all were fully susceptible to cefotaxime, ciprofloxacin and rifampicin.
When it came to vaccination against MenC, of all children with a known vaccination status, 26/32 (81.3%) were correctly vaccinated for their age, 4/32 (12.5%) had incomplete vaccination and 2/32 (6.2%) had not received any of the doses scheduled for their age. None were vaccinated against MenB. Information on vaccination in adult patients was only available in 2 cases, and 1 of these patients had been vaccinated.
The diagnosis was bacteraemia/sepsis in 47 cases (56%), meningitis in 24 (28.6%) and both in 13 (15.5%), without significant differences between children and adults. Table 2 compares the clinical presentation and laboratory findings of IMD in both age groups. Most patients presented with fever (96.4%). Purpuric rashes were more frequent in children (69% vs 29.4%; P=.006). A higher proportion of children required inotropic agents (42% vs 20.6%; P=.041).
Comparison of characteristics in children and adults.
| Total | Children | Adults | P | |
|---|---|---|---|---|
| n=84 (100%) | n=50 (59.5%) | n=34 (40.5%) | ||
| Demographic data | ||||
| Age (years) | 41.2 (26.4–69.3) | 2 (0.7–7.5) | 41.2 (26.4–69.3) | |
| Sex (male) | 47 (56%) | 33 (66%) | 14 (41.2%) | .024 |
| Confirmed cases | 72 (85.7%) | 41 (82%) | 31 (91.2%) | .238 |
| Clinical manifestations | ||||
| Diagnosis | ||||
| Meningitis | 24 (28.6%) | 15 (30%) | 9 (26.5%) | .942 |
| Bacteraemia | 47 (56%) | 26 (52%) | 21 (61.8%) | |
| Meningitis and bacteraemia | 13 (15.5%) | 9 (18%) | 4 (11.8%) | |
| Fever | 81 (96.4%) | 49 (98%) | 32 (94.1%) | .347 |
| Purpuric rash | 40 (47.6%) | 30 (69%) | 10 (29.4%) | .006 |
| Meningeal signs | 25 (29.8%) | 15 (30%) | 10 (29.4%) | .954 |
| Altered mental status | 37 (44%) | 22 (44%) | 15 (44.1%) | .991 |
| Low blood pressure | 36 (42.9%) | 24 (48%) | 12 (35.3%) | .248 |
| Hypoxaemia | 23 (27.4%) | 11 (22%) | 12 (35.3%) | .230 |
| Vomiting | 33 (39.3%) | 22 (44%) | 11 (32.4%) | .283 |
| Headache | 25 (29.8%) | 12 (24%) | 13 (38.2%) | .161 |
| Painful swallowing | 8 (9.5%) | 4 (8%) | 4 (11.8%) | .564 |
| Cough | 5 (6.0%) | 0 (0%) | 5 (14.7%) | .005 |
| Seizures | 2 (2.4%) | 2 (4%) | 0 (0%) | .238 |
| Treatment | ||||
| Oxygen therapy | 13 (15.5%) | 7 (14%) | 6 (17.6%) | .650 |
| Inotropic therapy | 28 (33.3%) | 21 (42%) | 7 (20.6%) | .041 |
| Endotracheal intubation | 22 (26.2%) | 11 (22%) | 11 (32.4%) | .289 |
| Dexamethasone | 29 (34.5%) | 17 (34%) | 12 (35.3%) | .670 |
| Third generation cephalosporins | 80 (95.2%) | 50 (100%) | 30 (88.2%) | .013 |
| Other antibiotics | 4 (4.8%) | 0 | 4 (11.8%) | .013 |
| Laboratory features | ||||
| Leukocytes (×109/L) | 12.5 (7.8–19) | 11.6 (7.8–21.4) | 13.7 (6.9–18.3) | .964 |
| Neutrophils (×109/L) | 10.6 (4.9–17.5) | 9.68 (3.6–18.3) | 13.1 (5.1–17.1) | .625 |
| Platelets (×109/L) | 191.5 (134.5–308.7) | 220 (142–327) | 173 (106.5–236.5) | .053 |
| CPR (mg/dL) | 10.7 (3.9–22.1) | 10.7 (3.7–21.2) | 11.1 (7.9–24) | .471 |
| Procalcitonin (ng/mL) | 12.3 (1.6–46.6) | 9.4 (2.7–36.5) | 25.5 (0.4–76.2) | .964 |
| aPTT (seg.) | 32.6 (29.1–41.4) | 33.9 (29.9–36.5) | 27.6 (25.9–32.7) | .007 |
| INR | 1.4 (1.2–1.7) | 1.5 (1.3–1.8) | 1.3 (1–1.4) | .026 |
aPTT, activated partial thromboplastin time; CPR, C-reactive protein.
Statistically significant differences are presented in boldface.
We have expressed categorical variables as absolute frequencies and percentages (%) and continuous variables as median and interquartile range.
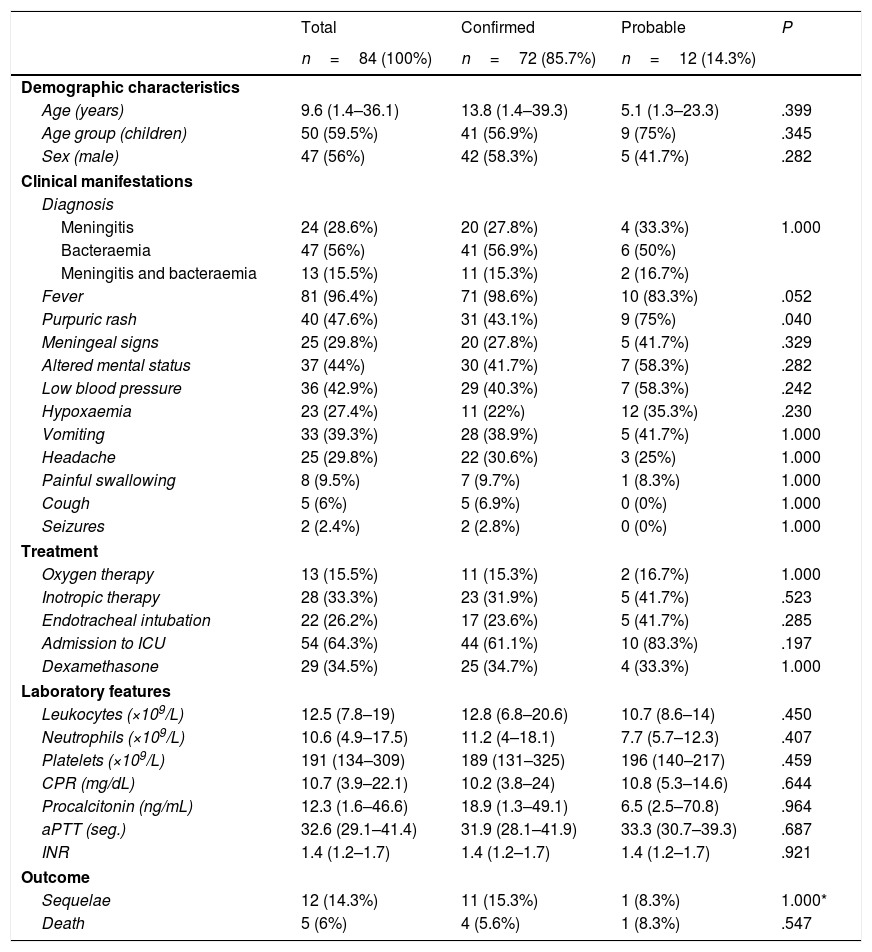
When we compared confirmed and probable cases (Table 3), the only difference we found was the higher proportion manifesting with a purpuric rash in the subset of patients with probable IMD (75% vs 43.1%; P=.040) and a higher prevalence of fever before admission in confirmed cases (98.6% vs 83.3%; .052).
Comparison of characteristics of confirmed versus probable cases.
| Total | Confirmed | Probable | P | |
|---|---|---|---|---|
| n=84 (100%) | n=72 (85.7%) | n=12 (14.3%) | ||
| Demographic characteristics | ||||
| Age (years) | 9.6 (1.4–36.1) | 13.8 (1.4–39.3) | 5.1 (1.3–23.3) | .399 |
| Age group (children) | 50 (59.5%) | 41 (56.9%) | 9 (75%) | .345 |
| Sex (male) | 47 (56%) | 42 (58.3%) | 5 (41.7%) | .282 |
| Clinical manifestations | ||||
| Diagnosis | ||||
| Meningitis | 24 (28.6%) | 20 (27.8%) | 4 (33.3%) | 1.000 |
| Bacteraemia | 47 (56%) | 41 (56.9%) | 6 (50%) | |
| Meningitis and bacteraemia | 13 (15.5%) | 11 (15.3%) | 2 (16.7%) | |
| Fever | 81 (96.4%) | 71 (98.6%) | 10 (83.3%) | .052 |
| Purpuric rash | 40 (47.6%) | 31 (43.1%) | 9 (75%) | .040 |
| Meningeal signs | 25 (29.8%) | 20 (27.8%) | 5 (41.7%) | .329 |
| Altered mental status | 37 (44%) | 30 (41.7%) | 7 (58.3%) | .282 |
| Low blood pressure | 36 (42.9%) | 29 (40.3%) | 7 (58.3%) | .242 |
| Hypoxaemia | 23 (27.4%) | 11 (22%) | 12 (35.3%) | .230 |
| Vomiting | 33 (39.3%) | 28 (38.9%) | 5 (41.7%) | 1.000 |
| Headache | 25 (29.8%) | 22 (30.6%) | 3 (25%) | 1.000 |
| Painful swallowing | 8 (9.5%) | 7 (9.7%) | 1 (8.3%) | 1.000 |
| Cough | 5 (6%) | 5 (6.9%) | 0 (0%) | 1.000 |
| Seizures | 2 (2.4%) | 2 (2.8%) | 0 (0%) | 1.000 |
| Treatment | ||||
| Oxygen therapy | 13 (15.5%) | 11 (15.3%) | 2 (16.7%) | 1.000 |
| Inotropic therapy | 28 (33.3%) | 23 (31.9%) | 5 (41.7%) | .523 |
| Endotracheal intubation | 22 (26.2%) | 17 (23.6%) | 5 (41.7%) | .285 |
| Admission to ICU | 54 (64.3%) | 44 (61.1%) | 10 (83.3%) | .197 |
| Dexamethasone | 29 (34.5%) | 25 (34.7%) | 4 (33.3%) | 1.000 |
| Laboratory features | ||||
| Leukocytes (×109/L) | 12.5 (7.8–19) | 12.8 (6.8–20.6) | 10.7 (8.6–14) | .450 |
| Neutrophils (×109/L) | 10.6 (4.9–17.5) | 11.2 (4–18.1) | 7.7 (5.7–12.3) | .407 |
| Platelets (×109/L) | 191 (134–309) | 189 (131–325) | 196 (140–217) | .459 |
| CPR (mg/dL) | 10.7 (3.9–22.1) | 10.2 (3.8–24) | 10.8 (5.3–14.6) | .644 |
| Procalcitonin (ng/mL) | 12.3 (1.6–46.6) | 18.9 (1.3–49.1) | 6.5 (2.5–70.8) | .964 |
| aPTT (seg.) | 32.6 (29.1–41.4) | 31.9 (28.1–41.9) | 33.3 (30.7–39.3) | .687 |
| INR | 1.4 (1.2–1.7) | 1.4 (1.2–1.7) | 1.4 (1.2–1.7) | .921 |
| Outcome | ||||
| Sequelae | 12 (14.3%) | 11 (15.3%) | 1 (8.3%) | 1.000* |
| Death | 5 (6%) | 4 (5.6%) | 1 (8.3%) | .547 |
aPTT, activated partial thromboplastin time; CPR, C-reactive protein.
Statistically significant differences are presented in boldface.
We have expressed categorical variables as absolute frequencies and percentages (%) and continuous variables as median and interquartile range.
A higher proportion of children required admission to the ICU compared to adults (78% vs 44.1%; P=.001). The mean duration of antibiotherapy was 9.8 days (IQR, 7–11 days), the mean length of stay was 10 days (IQR, 7–13 days), and the mean length of stay in the ICU was 2 days (IQR, 1–3.7 days), with no differences between children and adults. In the total sample, 34.5% of patients received dexamethasone as adjuvant therapy. There were 5 deaths (6% of the total sample). All of these patients died within 24h of admission. Mortality was higher in adult patients, although this difference was not statistically significant (4/34 [11.8%] vs 1/50 [2%], P=.153). Of these 5 patients, 3 had infection by MenB and 1 by a nontypeable strain, while N. meningitidis was not isolated in the remaining patient.
Of all survivors, 12/79 (15.2%) had sequelae after discharge, including 8/49 children (16.3%) and 4/30 adults (13.3%), with no differences based on age. The sequelae were cosmetic in 7 patients (8.3%), orthopaedic in 7 (8.3%), neurologic in 3 (3.6%) and renal in 3 (3.6%), and 1 patient had hearing loss (1.2%). In the subset of survivors, there were no significant differences in the development of sequelae between those infected by MenC (4/13; 30.8%) and those infected by MenB (7/31; 22.6%), while sequelae were significantly less frequent in patients with infection by untyped/nontypeable strains (1/35; 9%; P=.015 and 0.021 for the comparison with MenC and MenB, respectively).
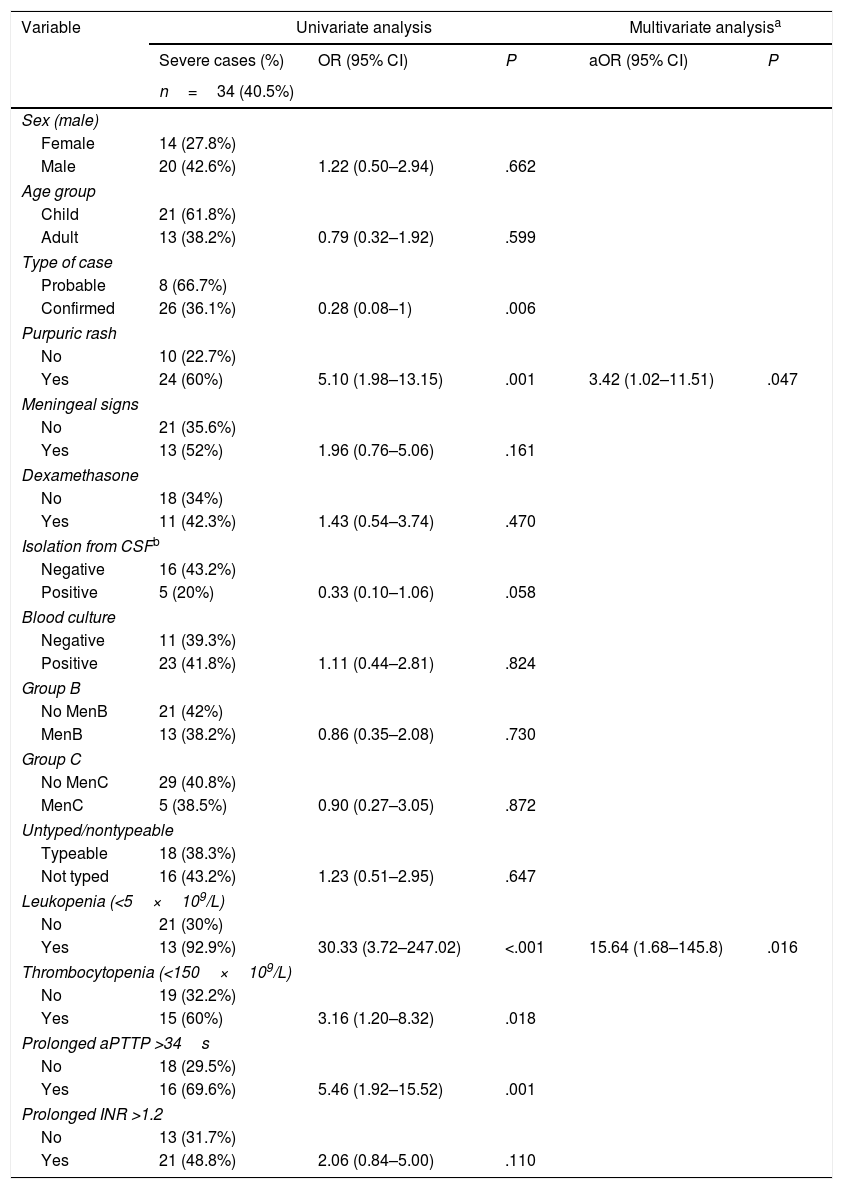
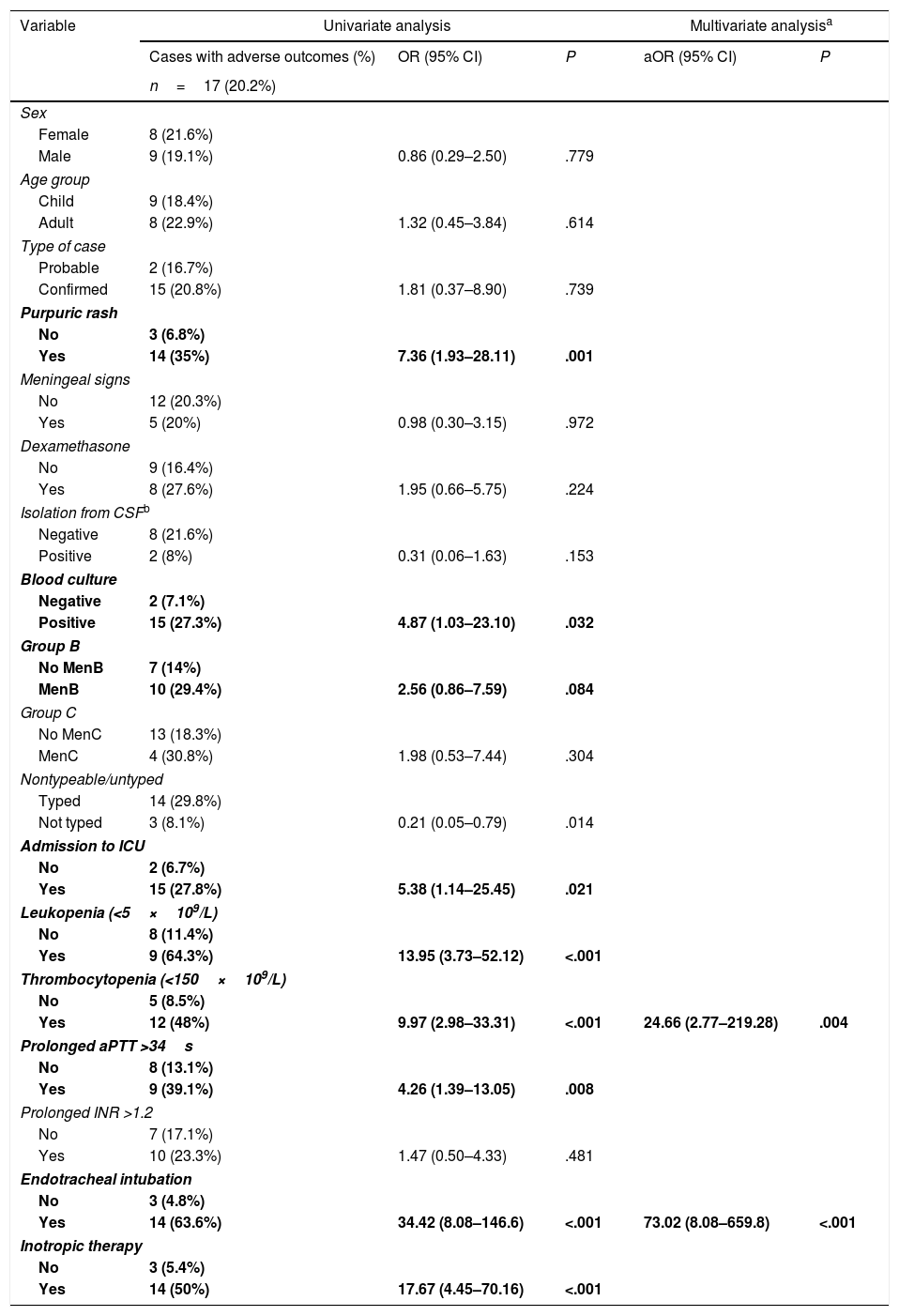
In the univariate analysis, the presence at diagnosis of a purpuric rash, a low WBC count, a high activated partial thromboplastin time or thrombocytopaenia were risk factors for severe disease and adverse outcome (Tables 4 and 5). Furthermore, isolation of N. meningitidis in blood culture and the need of inotropic therapy or ventilatory support were also risk factors for an adverse outcome. Severe disease was associated with an increased risk of sequelae (OR, 33.69; P<.001). In the multivariate analysis, a WBC count of less than <5×109cells/L (OR, 15.64; 95% confidence interval [CI], 1.68–145.8; P=.016) and the presence of a purpuric rash (OR, 3.42; 95% CI, 1.02–11.51]; P=.047) were independent risk factors for severe disease, while the need for endotracheal intubation (OR, 73.02; 95% CI, 8.08–659.8; P<.001) and thrombocytopaenia with a platelet count of less than 150×109/L (OR, 24.66; 95% CI, 2.77–219.28; P=.004) were independent risk factors for adverse outcome.
Univariate and multivariate analysis of factors associated with severity at diagnosis (need of mechanical ventilation and/or inotropic therapy).
| Variable | Univariate analysis | Multivariate analysisa | |||
|---|---|---|---|---|---|
| Severe cases (%) | OR (95% CI) | P | aOR (95% CI) | P | |
| n=34 (40.5%) | |||||
| Sex (male) | |||||
| Female | 14 (27.8%) | ||||
| Male | 20 (42.6%) | 1.22 (0.50–2.94) | .662 | ||
| Age group | |||||
| Child | 21 (61.8%) | ||||
| Adult | 13 (38.2%) | 0.79 (0.32–1.92) | .599 | ||
| Type of case | |||||
| Probable | 8 (66.7%) | ||||
| Confirmed | 26 (36.1%) | 0.28 (0.08–1) | .006 | ||
| Purpuric rash | |||||
| No | 10 (22.7%) | ||||
| Yes | 24 (60%) | 5.10 (1.98–13.15) | .001 | 3.42 (1.02–11.51) | .047 |
| Meningeal signs | |||||
| No | 21 (35.6%) | ||||
| Yes | 13 (52%) | 1.96 (0.76–5.06) | .161 | ||
| Dexamethasone | |||||
| No | 18 (34%) | ||||
| Yes | 11 (42.3%) | 1.43 (0.54–3.74) | .470 | ||
| Isolation from CSFb | |||||
| Negative | 16 (43.2%) | ||||
| Positive | 5 (20%) | 0.33 (0.10–1.06) | .058 | ||
| Blood culture | |||||
| Negative | 11 (39.3%) | ||||
| Positive | 23 (41.8%) | 1.11 (0.44–2.81) | .824 | ||
| Group B | |||||
| No MenB | 21 (42%) | ||||
| MenB | 13 (38.2%) | 0.86 (0.35–2.08) | .730 | ||
| Group C | |||||
| No MenC | 29 (40.8%) | ||||
| MenC | 5 (38.5%) | 0.90 (0.27–3.05) | .872 | ||
| Untyped/nontypeable | |||||
| Typeable | 18 (38.3%) | ||||
| Not typed | 16 (43.2%) | 1.23 (0.51–2.95) | .647 | ||
| Leukopenia (<5×109/L) | |||||
| No | 21 (30%) | ||||
| Yes | 13 (92.9%) | 30.33 (3.72–247.02) | <.001 | 15.64 (1.68–145.8) | .016 |
| Thrombocytopenia (<150×109/L) | |||||
| No | 19 (32.2%) | ||||
| Yes | 15 (60%) | 3.16 (1.20–8.32) | .018 | ||
| Prolonged aPTTP >34s | |||||
| No | 18 (29.5%) | ||||
| Yes | 16 (69.6%) | 5.46 (1.92–15.52) | .001 | ||
| Prolonged INR >1.2 | |||||
| No | 13 (31.7%) | ||||
| Yes | 21 (48.8%) | 2.06 (0.84–5.00) | .110 | ||
aOR, adjusted odds ratio (multivariate analysis); aPTT, activated partial thromboplastin time; CI, confidence interval; CSF, cerebrospinal fluid; INR, international normalised ratio; OR, odds ratio.
We have expressed categorical variables as absolute frequencies and percentages (%) and continuous variables as median and interquartile range.
Variables included in the multivariate analysis are presented in boldface.
Univariate and multivariate analysis of the factors associated with an adverse outcome (death and/or sequelae).
| Variable | Univariate analysis | Multivariate analysisa | |||
|---|---|---|---|---|---|
| Cases with adverse outcomes (%) | OR (95% CI) | P | aOR (95% CI) | P | |
| n=17 (20.2%) | |||||
| Sex | |||||
| Female | 8 (21.6%) | ||||
| Male | 9 (19.1%) | 0.86 (0.29–2.50) | .779 | ||
| Age group | |||||
| Child | 9 (18.4%) | ||||
| Adult | 8 (22.9%) | 1.32 (0.45–3.84) | .614 | ||
| Type of case | |||||
| Probable | 2 (16.7%) | ||||
| Confirmed | 15 (20.8%) | 1.81 (0.37–8.90) | .739 | ||
| Purpuric rash | |||||
| No | 3 (6.8%) | ||||
| Yes | 14 (35%) | 7.36 (1.93–28.11) | .001 | ||
| Meningeal signs | |||||
| No | 12 (20.3%) | ||||
| Yes | 5 (20%) | 0.98 (0.30–3.15) | .972 | ||
| Dexamethasone | |||||
| No | 9 (16.4%) | ||||
| Yes | 8 (27.6%) | 1.95 (0.66–5.75) | .224 | ||
| Isolation from CSFb | |||||
| Negative | 8 (21.6%) | ||||
| Positive | 2 (8%) | 0.31 (0.06–1.63) | .153 | ||
| Blood culture | |||||
| Negative | 2 (7.1%) | ||||
| Positive | 15 (27.3%) | 4.87 (1.03–23.10) | .032 | ||
| Group B | |||||
| No MenB | 7 (14%) | ||||
| MenB | 10 (29.4%) | 2.56 (0.86–7.59) | .084 | ||
| Group C | |||||
| No MenC | 13 (18.3%) | ||||
| MenC | 4 (30.8%) | 1.98 (0.53–7.44) | .304 | ||
| Nontypeable/untyped | |||||
| Typed | 14 (29.8%) | ||||
| Not typed | 3 (8.1%) | 0.21 (0.05–0.79) | .014 | ||
| Admission to ICU | |||||
| No | 2 (6.7%) | ||||
| Yes | 15 (27.8%) | 5.38 (1.14–25.45) | .021 | ||
| Leukopenia (<5×109/L) | |||||
| No | 8 (11.4%) | ||||
| Yes | 9 (64.3%) | 13.95 (3.73–52.12) | <.001 | ||
| Thrombocytopenia (<150×109/L) | |||||
| No | 5 (8.5%) | ||||
| Yes | 12 (48%) | 9.97 (2.98–33.31) | <.001 | 24.66 (2.77–219.28) | .004 |
| Prolonged aPTT >34s | |||||
| No | 8 (13.1%) | ||||
| Yes | 9 (39.1%) | 4.26 (1.39–13.05) | .008 | ||
| Prolonged INR >1.2 | |||||
| No | 7 (17.1%) | ||||
| Yes | 10 (23.3%) | 1.47 (0.50–4.33) | .481 | ||
| Endotracheal intubation | |||||
| No | 3 (4.8%) | ||||
| Yes | 14 (63.6%) | 34.42 (8.08–146.6) | <.001 | 73.02 (8.08–659.8) | <.001 |
| Inotropic therapy | |||||
| No | 3 (5.4%) | ||||
| Yes | 14 (50%) | 17.67 (4.45–70.16) | <.001 | ||
aOR, adjusted odds ratio (multivariate model); aPTT, activated partial thromboplastin time; CI, confidence interval; CSF, cerebrospinal fluid; INR, international normalised ratio; OR, odds ratio.
We have expressed categorical variables as absolute frequencies and percentages (%) and continuous variables as median and interquartile range.
Variables included in the multivariate analysis are presented in boldface. We excluded the variable “nontypeable/untyped serotype”, as it was associated with identification of group B and absence of isolation in culture (in the case of untyped strains), which were variables already included in the multivariate analysis.
Our study, conducted in a tertiary care hospital, reflects the shift in the epidemiology of IMD in Spain in the past few decades and has been the first in our country comparing the characteristics of IMD in children and adults. Between 2004 and 2017, the incidence of IMD in the population under study decreased by 50% in both children and adults. Of all cases, 59.5% occurred in the paediatric population, mainly in children aged less than 2 years, and MenB was the most prevalent serotype in children, compared to MenC in adults. The criteria for severe disease were met by 40.5% of cases, while 20.2% had an adverse outcome. The mortality was higher in the adult subset. The presence of purpuric rash, low WBC count and thrombocytopaenia at diagnosis were risk factors for having an adverse outcome.
The inclusion of vaccination against MenC in the routine immunisation schedule has played an essential role in the reduction of the incidence of IMD in Spain. In our sample, MenB was the predominant serogroup, although the proportion of cases of MenC was higher in adults (26.5% vs 8%; P=.022). Most children in the sample (84%) had received at least 1 dose of MenC vaccine. On the other hand, we were unable to determine the vaccination status of most of the adults. The higher incidence of MenC in the adult subset could be explained by a lower vaccination coverage in this age group. However, we could not test this hypothesis because the documentation of the vaccination history in most of the adult patients in the sample was incomplete.
The serogroup involved in most of the cases detected in our case series was MenB (40.5%). As observed in other studies,9 the decline in the number of cases caused by this serogroup occurred before the introduction of the vaccine in the immunisation schedule in 2015. This situation has already been described in the rest of Europe, where we are witnessing the lowest incidence of IMD due to MenB in the past 20 years.10 However, this serogroup characteristically emerges in epidemic waves, which, added to the considerable morbidity and mortality associated with IMD, underscores the importance of vaccination, even in periods with a low incidence.
In recent years, the incidence of IMD caused by serogroups Y and W has increased worldwide,11 accounting for up to 50% of cases of IMD in some European countries.12 This trend has also been observed in Spain, with a surge of cases caused by serogroup W,13 which increased 4-fold in the 2015–2016 season compared to the previous season.14 In our study, we found no cases caused by serogroups W or Y. However, since some of the strains were not available for serotyping, we cannot rule out the presence of these serotypes in our case series. We find the high percentage of cases in which N. meningitidis was isolated in culture without subsequent identification of the serotype surprising (24/84; 28.6%). One possible explanation is that the isolates were typed in the reference laboratory of the Centro Nacional de Microbiología but the results were not entered in the patients’ electronic health records.
The mortality of IMD ranges between 3.5% and 15% depending on the population and is higher in adults.8,9,15,16 In our study, the overall mortality was 6%, with a higher proportion in adults compared to children (11.8% vs 2%). These data are similar to those reported by a recent study conducted in Canada (overall mortality of 8.4%; 4% in children and 12% in adults).8 All deaths occurred within 24h of admission, which demonstrates the fulminant course of lethal cases already described in other studies, in which the deaths occurred in the first 48h.8,17
There is evidence that MenC is associated with more severe disease and a higher mortality,8 although in our case series none of the deaths were associated with this serogroup. In our population, thrombocytopaenia and endotracheal intubation were risk factors for an adverse outcome (death or sequelae). Several previous studies have analysed the presence of risk factors for a poor outcome and have reported an association with low WBC count,8,18–20 thrombocytopaenia,8,21 shock,8,18,20,22 coagulopathy,19,22,23 petechiae,18,20 ecchymotic rash8 or coma.17,22 The early identification of clinical or laboratory features associated with a poorer prognosis could be useful to identify patients requiring even closer monitoring.
Some studies have found better outcomes in patients managed in the ICU,8,24,25 although we found no evidence of this association in our case series. In our study, the proportion of patients admitted to the ICU was greater in the paediatric age group. Numerous studies have described an increase in survival when the initial stabilisation is optimal and shock management adequate.25,26 It is essential to ensure that the patient is closely monitored in the early stages of disease, and the patient should be admitted to the ICU if there is any indication of disease progression.27
One third of the patients received systemic corticosteroids at the beginning of treatment. Several guidelines recommend the use of steroid therapy in the management of bacterial meningitis.28 Contrary to the cases caused by Streptococcus pneumoniae or Haemophilus influenzae, in which the use of steroid therapy appears to improve outcomes,29 the usefulness of this treatment in cases caused by N. meningitidis remains under debate. Some experts30 recommend the use of corticosteroids in meningococcal meningitis due to the lower mortality found in several studies29 and the absence of significant associated risks. Nevertheless, the data available to date are not conclusive in demonstrating the benefits of administering corticosteroids to patients with meningococcal meningitis.
The proportion of patients that developed sequelae in our study was 15.6%, and there were no differences between age groups, contrary to the findings of other studies where sequelae were more frequent in children.8 In previous case series, the most frequent sequelae were neurologic (including hearing loss), skin scarring and amputation of extremities.31,32 These findings are similar to those of our study, in which cosmetic sequelae were most frequent. However, only 1 patient in our sample had hearing loss.
There are limitations to our study. Its retrospective design impeded the rigorous collection of data for all analysed variables, including the vaccination status of most adults. Due to the small number of cases, the analysis did not have sufficient statistical power to establish the association between severity of IMD and potential risk factors or to perform analysis of specific sample subsets. Furthermore, the collection of data in a single centre provides a limited perspective on the global situation of IMD in Spain. The main strength of the study is that it included and analysed together the paediatric and adult populations, which allowed a comparison of the differential characteristics in both groups.
In conclusion, the incidence of IMD has decreased in our region in recent decades, and MenB is the most prevalent serogroup. Invasive meningococcal disease continues to be more frequent in children, especially infants younger than 1 year, in whom infection by MenB is most prevalent. In opposition, in the adult population, infection by MenC is more frequent, probably due to a lower vaccination coverage in this age group. Despite advances in its diagnosis and treatment, IMD can have a fulminant course and cause serious sequelae, which demands improvement in strategies for prevention and early detection. The detection of risk factors at onset, such as thrombocytopaenia, a low WBC count or a purpuric rash, can help identify patients at increased risk of adverse outcomes.
FundingThis project was not funded by any specific grants from the public sector or for-profit or non-profit nongovernmental organisations.
Conflicts of interestThe authors have no conflicts of interest to declare
Begoña Santiago-García: Section of Paediatric Infectious Diseases, Department of Paediatrics Hospital General Universitario Gregorio Marañón, Madrid (Spain).
Jesús Saavedra-Lozano: Section of Paediatric Infectious Diseases, Department of Paediatrics Hospital General Universitario Gregorio Marañón, Madrid (Spain).
Mar Santos: Section of Paediatric Infectious Diseases, Department of Paediatrics Hospital General Universitario Gregorio Marañón, Madrid (Spain).
Emilia Cercenado: Department of Clinical Microbiology and Infectious Diseases. Hospital General Universitario Gregorio Marañón, Madrid (Spain).
Please cite this article as: Maturana Martínez D, Aguilera-Alonso D, García Mancebo J, Navarro ML, Hernández Sampelayo T, Rincón López EM. Enfermedad meningocócica invasiva en niños y adultos en un hospital terciario: epidemiología reciente y factores pronósticos. An Pediatr (Barc). 2019;91:296–306.
Appendix A lists the members of the Study Group on Invasive Meningococcal Disease and the institutions they are affiliated to.
Previous presentations: This study was presented at the IX Congress of the Sociedad Española de Infectología Pediátrica and the VIII Reunión Hispano-Mexicana de Infectología Pediátrica, held March 8–10, 2018 in Seville, Spain, and the 35th Annual Meeting of the European Society for Paediatric Infectious Diseases (ESPID 2017) held May 23–27, 2017 in Madrid, Spain.