Invasive group A streptococcal disease (iGASD) is a serious infection in children. Several studies have shown an increased incidence in the past years.
ObjectiveTo evaluate the characteristics and outcome of iGASD in children, and to determine changes in incidence or severity.
Material and methodsA retrospective study was conducted on children ≤16 years evaluated in a tertiary paediatric hospital in Madrid, and diagnosed with iGASD (June 2005–July 2013). An analysis was made of the demographics, symptomatology, microbiology, and treatment. The changes throughout the period studied were evaluated, as well as parameters associated with disease severity.
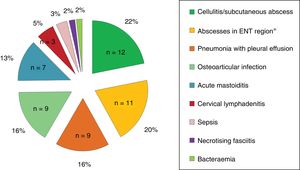
ResultsThe study included a total of 55 children with iGASD, with 33 (60%) females, and a median age of 48.5 (20.5–88.9) months. The most frequent clinical syndromes were cellulitis/subcutaneous abscess (21.8%), ENT abscess (20%), pneumonia (16.4%), osteoarticular infection (16.4%), and mastoiditis (12.7%). The incidence of iGASD (cases/105emergencies/year) increased from 5.6 (4.2–7.2) between June 2005 and May 2009 to 18.9 (15.1–26) between June 2009 and May 2013; P=.057. Surgery and admission to PICU was required by 35 (63.6%) and 10 (18.2%) patients, respectively. Children in PICU were younger (26.5 months vs 52.6 months, P=.116), had a higher C-reactive protein (24.5mg/dl vs 10.7mg/dl, P<.001) and higher frequency of pneumonia (60% vs 7%, P<.001). In the multivariate analysis, only C-reactive protein was a risk factor for admission to PICU (OR: 1.14 [1.004–1.286], P=.04). There were no sequelae.
ConclusionsAn increased incidence of iGASD was observed in the children in this study. Lower age, pneumonia, and higher C-reactive protein were associated with disease severity in this series.
La enfermedad invasiva por Streptococcus del grupo A (EISGA) es una infección grave en niños, habiéndose comunicado un aumento de incidencia en los últimos años.
ObjetivoEvaluar las características y evolución de la EISGA en niños y determinar cambios en la incidencia o gravedad.
Material y métodosEstudio retrospectivo de niños ≤16 años evaluados en un hospital terciario pediátrico de Madrid y diagnosticados de EISGA (junio 2005-julio 2013). Se analizó la epidemiología, clínica, microbiología y tratamiento, evaluándose cambios a lo largo del periodo estudiado y parámetros asociados a gravedad.
ResultadosSe incluyeron 55 niños con EISGA; 33 (60%) mujeres, con una mediana de 48,5 (20,5-88,9) meses. Los síndromes clínicos más frecuentes fueron celulitis/absceso subcutáneo (21,8%), absceso ORL (20%), neumonía (16,4%), infección osteoarticular (16,4%) y mastoiditis (12,7%). La incidencia de EISGA (casos/105urgencias/año) aumentó de 5,6 (4,2-7,2) entre junio 2005-mayo 2009 a 18,9 (15,1-26) entre junio 2009-mayo 2013; p=0,057. El 63,6% (n=35) y el 18,2% (n=10) de los pacientes precisaron cirugía e ingreso en UCIP, respectivamente. Los niños en UCIP fueron más pequeños (26,5 vs. 52,6 meses; p=0,116), presentaron proteína C reactiva más elevada (24,5 vs. 10,7mg/dl; p<0,001) y mayor frecuencia de neumonía (60 vs. 7%; p<0,001). En el análisis multivariante solo la proteína C reactiva fue factor de riesgo de ingreso en UCIP (OR: 1,14 [1,004-1,286]; p=0,04). No hubo secuelas.
ConclusionesSe objetivó un aumento de la incidencia de EISGA en niños en nuestro medio, siendo la menor edad, la presencia de neumonía y la proteína C reactiva elevada los parámetros asociados a gravedad en esta serie.
Group A β-haemolytic streptococci (GABHS) are gram-positive, facultative anaerobic streptococci that are a frequent cause of disease in children. They are present in a large proportion of individuals in the oropharynx and skin1 and may colonise these regions asymptomatically or causing diseases, usually not invasive, such as tonsillitis, scarlet fever or impetigo.2 However, at times GABHS may cause severe invasive disease associated with a high morbidity and mortality.3
In the past 30 years, there has been an increase in the incidence of invasive infection by GABHS in different geographical regions,4–10 including cases of very severe disease,2 the cause of which is not well understood.1,5,10–14 In 2002, 11 European countries started a project for the investigation and surveillance of invasive disease by GABHS (iGASD), that was named Strep-EURO whose goal was to elucidate the epidemiology of this disease in the European continent. Thus, it is known that in Europe the incidence of iGASD ranges between 0.4 and 4.8 cases per 100000 individuals per year, while in the United States it is estimated at 3.54 cases per 100000 individuals per year, with a mortality rate in children of 0–14%. Spain did not participate in the Strep-EURO project, and since iGASD is not a notifiable disease, its incidence in our country is not known.6 In addition, there are other aspects that have not been established in regard to iGASD in children in Spain, such as its epidemiological and clinical characteristics or the differences in comparison with the same disease in adults.
The objectives of our study were: (1) to describe the epidemiological, clinical and microbiological characteristics of iGASD in children treated in a tertiary care hospital over a period of 8 years, as well as to determine risk factors for severe disease and (2) to analyse potential changes in the incidence and severity of this disease in our geographical area during the period under study.
Materials and methodsStudy designWe conducted a descriptive, retrospective study in children aged 16 years or less assessed in a tertiary care children's hospital in Madrid that were given a diagnosis of iGASD over a period of 8 years and 2 months (June 2005–July 2013). We analysed the cases of those children with microbiological confirmation of the diagnosis of iGASD according to the criteria established by the Working Group on Severe Streptococcal Infections: detection of GABHS in a sample of a normally sterile body site, with or without clinical manifestations of invasive disease.15 We excluded patients in whom isolation of GABHS was not confirmed or other aetiological agents were identified. The isolation, identification and antibiotic susceptibility testing of GABHS were performed in adherence to customary microbiological protocols.16 We identified cases through the review of health records in the hospital discharge coding databases following the protocol established by the hospital and by reviewing the sample records of the Department of Microbiology.
Data collectionWe collected epidemiological, clinical and laboratory data through the review of health records. We analysed the following variables: (1) demographic and epidemiological variables, including the vaccination status and underlying diseases; (2) clinical variables, such as fever, manifestations at diagnosis and risk factors for iGASD described in the previous literature5,14,17–19 (such as history of varicella or surgical intervention, hospital admission in the past 3 months, acute pharyngotonsillitis in the past 6 months or invasive infection in the past 12 months) and antibiotherapy prescribed previous to hospital care in an outpatient setting, during management in hospital and after discharge; (3) laboratory variables, such as total white blood cell count, percentage of neutrophils, serum level of C-reactive protein (PCR, normal range<0.9mg/dL) and serum level of procalcitonin (normal range<0.3ng/mL); (4) microbiological data, such as diagnostic tests (culture, PCR with universal primers for gene 16SrARN and gene sequencing [PCR16S] or rapid antigen detection) and antimicrobial susceptibility testing; (5) outcome variables, including the need and duration of admission to the paediatric intensive care unit (PICU), total length of stay, need for surgery, sequelae and mortality associated with iGASD.
Analysis of the incidence in the period under studyTo evaluate potential changes in the characteristics of iGASD throughout the period under study, we divided it in 2 time periods, each lasting 48 months, for the purpose of comparison: P1 (June 2005–May 2009) and P2 (June 2009–May 2013). To do so, and only in this particular analysis, we excluded 2 patients that received the diagnosis between June 1 and July 31, 2013. In addition, when we analysed the incidence, we excluded years 2005 and 2013, as we did not have data for every month. To calculate the annual incidence, we used the number of children assessed at the paediatric emergency department of the hospital each year, as we considered it a more objective parameter compared to the entire catchment population of the hospital.
Statistical analysisWe present demographic and epidemiological characteristics using descriptive statistics. We have expressed quantitative variables as mean±standard deviation (SD) or median and interquartile range (IQR, 25th–75th percentile) depending on whether the data followed or not a normal distribution, and qualitative variables as percentages. To compare qualitative data we used the chi square test or the Fisher exact test, and to compare quantitative data the Student t test or Mann–Whitney U test depending on the shape of the distribution. We analysed correlations by means of the Spearman test. We defined statistical significance as a P-value of less than 0.05. We performed the statistical analyses with the software SigmaPlot 11.0 (Systat Software, Inc; San Jose, CA, USA).
ResultsEpidemiological dataThe study included a total of 55 children with a diagnosis of iGASD; 33 were female (60%), and the median age was 48.5 months (IQR, 20.5–88.9). Most patients (87.3%) were previously healthy. Twenty percent had at least one risk factor for iGASD, most frequently acute pharyngotonsillitis in the past 6 months (14.5% of the total). Other risk factors (each of them found in 1 patient) were varicella infection in the past month and a previous history of an open wound or surgery.
Clinical characteristics and laboratory findings at the time of diagnosisOf all children, 76.4% had fever at diagnosis, and less frequently painful swallowing (18.2%), skin involvement (16.4%), displacement of the pinna (12.7%), difficulty breathing (10.9%) and arthralgia (10.9%). Twenty-four percent of the children had received antibiotherapy prior to coming to hospital. Fig. 1 presents the forms of disease most frequently associated with invasive infection by GABHS.
A complete blood count was performed at admission in 49 patients, with a median white blood cell count of 16100cells/μL (IQR, 10478–23725) and 75.5% neutrophils (IQR, 69.3%–87.3%). The serum levels of CRP were measured in 42/55 patients and procalcitonin levels in 7/55 patients. The median level of CRP was 11.5mg/dL (IQR, 6.3–21.6), and the mean level of procalcitonin was 3.1ng/mL (SD, ±3.1).
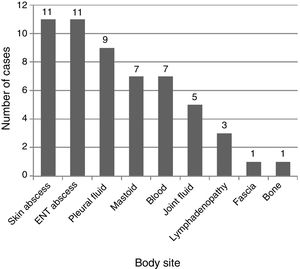
Fig. 2 shows the anatomical sites from which the samples of the different microbiological isolates were obtained. The method used most frequently for aetiological diagnosis was conventional culture (n=47; 85.4%), followed by PCR 16S (n=7; 12.7%) and antigen detection (n=1; 1.8%; pleural fluid sample). Forty-three percent of the children in whom GABHS was detected by means of 16S PCR (3/7) had received antibiotics before diagnosis. Antimicrobial susceptibility testing was performed for 48 isolates, and 100% were sensitive to penicillin, and only 1 isolate exhibited resistance to both clindamycin and macrolide antibiotics.
Treatment, clinical outcomes and complicationsOf all the patients in the sample, 50 (91%) were admitted to hospital and the rest managed at the outpatient level. We obtained information about antibiotic treatment for 52 children (94.5%), of who 47 (90.4%) required intravenous (IV) antibiotherapy for a median duration of 8 days (IQR, 4.2–13). The most frequently prescribed IV antibiotic was amoxicillin–clavulanic acid (40.4%), followed by cefotaxime (23.4%) and cefotaxime combined with clindamycin (10.6%). In total, 6 children (11.3%) received clindamycin in combination with another antibiotic. Forty-seven of the 52 patients (90.4%) received a prescription for oral antibiotherapy after discharge, for a median duration of 7 days (IQR, 7–10), and the antibiotics prescribed most frequently after discharge were amoxicillin–clavulanic acid (51.1%) and amoxicillin (36.2%) The median total duration of antibiotherapy (IV and oral) was of 15 days (IQR, 11–21).
We defined severe iGASD as that requiring admission to the PICU (10/55; 18.2%). Of these 10 children, 30% required inotropic support and 20% mechanical ventilation. The mean length of stay in the PICU was 5.4 days (SD, ±4.2). We also analysed several variables to determine the presence of potential risk factors for admission to the PICU, and found that these patients were younger (26.5 months vs 52.6 months; P=.12), had a higher percentage of neutrophils (88.5% vs 74.7%; P=.001) and greater elevation of CRP (24.5mg/dL vs 10.7mg/dL; P=.001) (Table 1). Furthermore, patients with pneumonia and pleural effusion were more likely to require admission to the PICU (odds ratio [OR], 21 [3.74–117.76]; P=.001). In the multivariate analysis, the only variable that was a significant risk factor for admission to the PICU was the CPR level (OR, 1.14 [1.004–1.29]; P=.04). None of the patients died or experienced sequelae.
Epidemiological, laboratory and clinical characteristics of patients admitted and not admitted to the PICU (n=55).
| Admitted to PICU (n=10) | Not admitted to PICU (n=45) | P | |
|---|---|---|---|
| Epidemiological characteristics | |||
| Age (months) | 26.5 [19.1–53] | 52.6 [22.8–118.9] | .116 |
| Female sex [n (%)] | 8 (80) | 25 (54.5) | .284 |
| Presence of underlying disease [n (%)] | 0 (0) | 7 (15.5) | .328 |
| Clinical characteristics | |||
| Fever | |||
| Presence [n (%)] | 8 (80) | 34 (75.5) | 1 |
| Maximum temperature (°C) | 39.4 [38.5–39.6] | 38.5 [38–39] | .181 |
| Diagnosis of iGASD [n (%)] | <.001 | ||
| Pneumonia with pleural effusion | 6 (60) | 3 (6.7) | |
| Peritonsillar abscess | 1 (10) | 9 (20) | |
| Retropharyngeal abscess | 1 (10) | 0 (0) | |
| Necrotising fasciitis | 1 (10) | 0 (0) | |
| Sepsis | 1 (10) | 1 (2.2) | |
| Cellulitis/skin abscess | 0 (0) | 12 (26.7) | |
| Arthritis | 0 (0) | 8 (17.8) | |
| Mastoiditis | 0 (0) | 7 (15.5) | |
| Lymphadenitis | 0 (0) | 3 (6.7) | |
| Bacteraemia | 0 (0) | 1 (2.2) | |
| Osteomyelitis | 0 (0) | 1 (2.2) | |
| Laboratory characteristics | |||
| Leukocytes (cells/μL) | 11900 [5100–23700] | 17600 [12375–23700] | .249 |
| Neutrophils (%) | 88.5 [84.2–93.6] | 74.7 [67–85.3] | .001 |
| CRP (mg/dL) | 24.5±9.5 | 10.7±7.8 | <.001 |
| Clinical outcome | |||
| Length of stay (days) | 14 [6–26] | 7 [4–11.5] | .024 |
| Need of surgery [n (%)] | 6 (60) | 29 (64.4) | 1 |
| Days of IV ABXa | 15.5 [6–23] | 7 [3–10] | .013 |
| Total days ABXb | 19.5 [14–23] | 15 [10–20] | .061 |
| Oral A/C at discharge [n (%)] | 4 (40) | 21 (46.7) | .741 |
ABX, antibiotherapy; A/C, amoxicillin–clavulanic acid.
Data expressed as median and interquartile range or mean±standard deviation. The numbers in parenthesis represent the percentage of patients in each group.
Statistically significant results are presented in boldface (P<.05).
Of all patients, 63.6% (n=35) required surgery; these patients were older (61 months vs 29 months; P=.07), had longer lengths of stay and required longer courses of antibiotherapy (18 days vs 13 days; P=.044). In addition, prescription of amoxicillin–clavulanic acid at discharge in this group was less frequent (39% vs 81%; P=.007). We did not find any statistically significant differences in the rest of the variables under study (Table 2).
Epidemiological, laboratory and clinical characteristics of patients that did and did not undergo surgical intervention (n=55).
| Patients that underwent surgery (n=35) | Patients that did not undergo surgery (n=20) | P | |
|---|---|---|---|
| Epidemiological characteristics | |||
| Age (months) | 60.7 [25.8–119] | 29.1 [14.7–61.7] | .066 |
| Female sex [n (%)] | 21 (60) | 12 (60) | 1 |
| Presence of underlying disease [n (%)] | 5 (14.3) | 2 (10) | 1 |
| Clinical characteristics | |||
| Fever | |||
| Presence[n (%)] | 26 (74.3) | 16 (80) | .749 |
| Maximum temperature (°C) | 38.6 [38.3–39.6] | 38.6 [38–39] | .405 |
| Diagnosis of iGASD [n (%)] | .144 | ||
| Pneumonia with pleural effusion | 7 (20) | 2 (10) | |
| Peritonsillar abscess | 8 (22.8) | 2 (10) | |
| Retropharyngeal abscess | 1 (2.8) | 0 (0) | |
| Necrotising fasciitis | 1 (2.8) | 0 (0) | |
| Sepsis | 0 (0) | 2 (10) | |
| Cellulitis/skin abscess | 5 (14.3) | 7 (35) | |
| Arthritis | 7 (20) | 1 (5) | |
| Mastoiditis | 3 (8.6) | 4 (20) | |
| Lymphadenitis | 2 (5.7) | 1 (5) | |
| Bacteraemia | 0 (0) | 1 (5) | |
| Osteomyelitis | 1 (2.8) | 0 (0) | |
| Laboratory characteristics | |||
| Leukocytes (cells/μL) | 15950 [9750–23550] | 16100 [13050–24550] | .508 |
| Neutrophils (%) | 78.4 [70.4–86] | 74.7 [67.3–90.9] | .908 |
| CRP (mg/dL) | 11.5 [6.3–19.1] | 12.1 [6.7–24.2] | .660 |
| Clinical outcome | |||
| Length of stay (days) | 9 [5–15] | 6 [4–8] | .074 |
| Admission to PICU [n (%)] | 6 (17.1) | 4 (20) | 1 |
| Days of IV ABXa | 8 [5–15] | 5 [4–6] | .029 |
| Total days ABXb | 17.5 [14–22] | 12.5 [10–16] | .044 |
| Oral A/C at discharge [n (%)] | 12 (38.7) | 13 (81.2) | .007 |
ABX, antibiotherapy; A/C, amoxicillin–clavulanic acid.
Data expressed as median and interquartile range or mean±standard deviation. The numbers in parenthesis represent the percentage of patients in each group.
Statistically significant results are presented in boldface (P<.05).
To assess whether age may have an impact in the outcome of iGASD, we compared patients aged less and more than 48 months, and found that the younger subset had required surgery less frequently (51.8% vs 75%; P=.097), had longer lengths of stay (9.5 days vs 7 days; P=.073), received IV antibiotherapy more frequently (100% vs 73.1%; P=.01) and had longer total durations of antibiotherapy (17.3 days vs 13.5 days; P=.026). A greater proportion of children aged 48 months or older developed peritonsillar abscesses (OR, 11.8 [1.4–101.7]; P=.01).
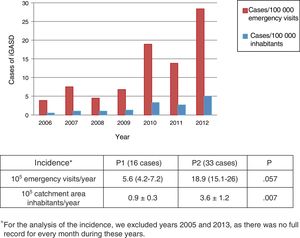
Differences in the incidence and clinical characteristics of children with iGASD through the period under studyWe found that the incidence of iGASD increased during the period under study (Table 3 and Fig. 3).
Annual incidence of iGASD based on number of emergency visits and the catchment population of the hospital.
| Year | Number of iGASD cases | Number of emergency visits | Incidence in emergency patientsa | Population (approx.)b | Incidence in populationc |
|---|---|---|---|---|---|
| 2006 | 3 | 76386 | 3.9 | 600000 | 0.5 |
| 2007 | 6 | 79782 | 7.5 | 600000 | 1 |
| 2008 | 3 | 66142 | 4.5 | 300000 | 1 |
| 2009 | 4 | 58583 | 6.8 | 300000 | 1.3 |
| 2010 | 10 | 52964 | 18.9 | 300000 | 3.3 |
| 2011 | 8 | 57766 | 13.8 | 300000 | 2.7 |
| 2012 | 15 | 52836 | 28.4 | 300000 | 5 |
To exclude the possibility that this increase in incidence was due to an increase in the detection of bacteria in blood cultures, we analysed the detection rate for Escherichia coli and Staphylococcus aureus during the years under study, and did not find evidence of an increase in the number of isolations per 1000 blood cultures per year between P1 and P2 (personal communication).
Last of all, we compared the different epidemiological, laboratory, severity and outcome parameters in the patients in the 2 periods established for analysis (P1 and P2), and found no significant differences between the periods in any of the variables (Table 4).
Epidemiological, laboratory and clinical characteristics by period under study (n=53)a
| P1 (June 2005–May 2009) (n=16) | P2 (June 2009–May 2013) (n=37) | P | |
|---|---|---|---|
| Epidemiological characteristics | |||
| Age (months) | 56.6 [13.5–80.1] | 43.4 [24.7–108.7] | 0.72 |
| Female sex [n (%)] | 8 (50) | 24 (64.9) | 0.368 |
| Presence of underlying disease [n (%)] | 1 (6.2) | 5 (13.5) | 0.655 |
| Laboratory characteristics | |||
| Leukocytes (cells/μL) | 14300 [13050–23350] | 16500 [9600–23700] | 0.748 |
| Neutrophils (%) | 75.5 [72.1–92.5] | 75 [68–86] | 0.385 |
| CRP (mg/dL) | 9.8 [5.1–19.3] | 11.6 [8–21.9] | 0.679 |
| Clinical characteristics | |||
| Fever | |||
| Present [n (%)] | 11 (68.7) | 30 (81.1) | 0.475 |
| Maximum temperature (°C) | 38.8 [38.1–39] | 38.5 [38–39.5] | 0.940 |
| Diagnosis of iGASD [n (%)] | 0.722 | ||
| Cellulitis/skin abscess | 3 (18.7) | 9 (24.3) | |
| Peritonsillar abscess | 3 (18.7) | 7 (18.9) | |
| Pneumonia with pleural effusion | 3 (18.7) | 6 (16.2) | |
| Mastoiditis | 3 (18.7) | 4 (10.8) | |
| Arthritis | 3 (18.7) | 3 (8.1) | |
| Lymphadenitis | 0 (0) | 3 (8.1) | |
| Sepsis | 1 (6.2) | 1 (2.7) | |
| Bacteraemia | 0 (0) | 1 (2.7) | |
| Necrotising fasciitis | 0 (0) | 1 (2.7) | |
| Retropharyngeal abscess | 0 (0) | 1 (2.7) | |
| Osteomyelitis | 0 (0) | 1 (2.7) | |
| Microbiological diagnosis | |||
| Technique used[n (%)] | 0.062 | ||
| Conventional culture | 15 (93.7) | 30 (81.1) | |
| PCR | 0 (0) | 7 (18.9) | |
| Antigen test | 1 (6.2) | 0 (0) | |
| Clinical outcome | |||
| Length of stay (days) | 7 [3–15] | 7.5 [4–13] | 0.982 |
| Admission to PICU [n (%)] | 3 (18.7) | 7 (18.9) | 1 |
| Days of IV ABXb | 7 [3–13] | 7 [4–12] | 0.778 |
| Total days of ABXc | 16 [10–23] | 15 [11–19.5] | 0.522 |
| Oral A/C at discharge [n (%)] | 5 (31.2) | 20 (54) | 0.22 |
ABX, antibiotherapy; A/C, amoxicillin–clavulanic acid.
Data expressed as median and interquartile range or mean±standard deviation. The numbers in parenthesis represent the percentage of patients in each group.
The main findings of the study were the following: (1) we found an increase in the incidence of iGASD in children in our area in the past few years; (2) this observed increase in incidence was not associated with any other changes in epidemiological, microbiological or clinical parameters related to iGASD; (3) the severity of iGASD was considerable, with a high percentage of patients requiring admission to the PICU and surgical intervention, and the risk factors associated with increased severity were age less than 48 months, diagnosis of pneumonia with pleural effusion and elevation of CPR; and (4) the rate of resistance of GABHS to macrolides and clindamycin remained low throughout the period under study.
The incidence of iGASD seems to have been increasing in the past few years worldwide, as evinced by recent studies conducted in children10,12,18 and adults.3,7,18,19 However, the epidemiology of iGASD is not well known in most countries, including Spain, as there are no adequate surveillance systems in place,14 so that the data on its incidence are limited, especially in the paediatric population. In our case series, the incidence tripled from the beginning to the end of the study period, increasing from 5.6 to 18.9 cases per 105 paediatric emergency visits per year, which diverged from the findings of a recent study in a children's hospital in Barcelona, where there was no increase in the incidence of iGASD in the 6 years included in the analysis.20. The incidence in P2 was of 3.6 cases per 105 inhabitants per year, which was very similar to the incidence found by Montes et al.6 in Spain (3.11 cases/105 inhabitants), but lower compared to the incidence reported for the United States (5.3 cases/105 inhabitants/year), although it was greater compared to the mean found in other European countries, such as Sweden (2.1 cases/105 inhabitants in 2012)1 or Finland (2.5 cases/105 inhabitants in the 2006–2010 period).10
The causes of this increase in the incidence of iGASD are not well understood, but increases in risk factors or improvement in microbiological methods may be among them. Varicella has classically been one of the most important risk factors for development of iGASD in children,2,10,21 especially in those that experience a severe invasive cutaneous infection.22 However, our study and other recent works20,23 have not found evidence of an increase in the incidence of varicella, consistent with improvements in the coverage of vaccination against this disease.24 There is evidence that the diagnostic yield of molecular techniques is higher for certain microorganisms compared to conventional diagnostic methods, especially in patients previously treated with antibiotics.25,26 There has also been a proven increase in the accuracy of diagnosis of pneumonia with empyema caused by GABHS in children, both with the use of genetic techniques27 as well as antigen detection methods.28,29 In our hospital, 16S PCR was introduced in 2009, and 19% of the diagnosis of iGASD in P2 were made with this technique. Since many of the infections were suppurative and at least 43% of the children in whom diagnosis was achieved by means of 16S PCR had previously taken antibiotics, the use of molecular diagnostic methods may have played an important role in the increase in diagnosis of iGASD. However, we did not find any clear change in specific clinical forms nor in the isolation of other bacteria, nor epidemiological differences between the 2 periods, so the increase in incidence may be related to other factors that we did not analyse. Thus, numerous studies have found an increase in the severity of iGASD caused by certain genotypes in children,6,10,14,23,30–32 while other authors have reported that the incidence of iGASD may be associated with the susceptibility of specific subpopulations to those strains.33
The most frequent form of iGASD observed in our study was skin and soft tissue infection, which was consistent with many previous studies.6,10,20,34 However, overall, in our case series infections were most frequently located in the ear–nose–throat region. Contrary to other case series, we only found 1 case of primary bacteraemia,10,14,24,34,35 1 case of necrotising fasciitis and no deaths,10,20,32 which could be attributed to the low incidence of varicella and differences between age groups in clinical presentation (fewer cases of necrotising fasciitis and septic shock in children aged less than 10 years)33,36 or to technological advances in the PICU and the multidisciplinary management of these patients.23
Invasive infection by GABHS can cause significant morbidity and a high proportion of affected patients may require surgery and/or admission to the PICU. Our study, one of the largest conducted in Spain, contributes objective data on the severity of iGASD in children in our region. Thus, we found that 18% of patients with iGASD were admitted to the PICU and 63.5% required some form of surgical intervention. These data were consistent with those of previously published paediatric case series, in which the proportion admitted to the PICU ranged between 19% and 57%10,24,35 and the proportion that required surgery between 43% and 64%.10,24,32,35 However, in the study conducted in Barcelona, despite the geographical proximity, the proportion admitted to the PICU was significantly greater (32.7%), while the proportion that underwent surgery was significantly lower (34.6%) compared to our case series in Madrid,20 which could be explained by the higher proportion of children with chronic skin diseases. We ought to underscore that both younger age and the presence of pneumonia were predictors of severe disease in our study, which was consistent with the findings of other authors,5,18,23,24 which suggests that when iGASD is diagnosed in younger children or children with pneumonia, clinicians should contemplate a more aggressive approach to management. Elevation of CPR at admission was another independent risk factor for admission to the PICU, which was also consistent with previous studies.13,20,32 Despite the considerable severity of the disease, it does not seem as if the clinical presentation or the outcomes of iGASD have changed in our region in recent years.
Last of all, in our series we found a very low rate of macrolide and clindamycin resistance in GABHS compared to other studies.6,17 Although antibiotic use in Spain may be high,37 considerable efforts are being made to develop protocols, strategic plans and training programmes for the rational use of antimicrobials.38,39 In this regard, in our study we found that children that required surgery frequently received a narrow-spectrum oral antibiotic compared to children that did not require surgery. We did not find a clear reason for this difference, although it may suggest a different approach to the use of antibiotics.
Some of the limitations of our study are its retrospective design, the small sample size and that it was conducted in a single centre, which limits its statistical power and hinders the generalisation of its result. Furthermore, tests for serotyping or determining the virulence of GABHS strains were not performed, so we could not know whether these factors changed over the period under study or could have influenced the severity of iGASD. Nevertheless, our study is still the largest study on iGASD in children conducted in Spain to date along with the one published by Arias-Constantí et al.,20 and it provides valuable data on the current situation of this disease in Spain, which can help design future studies to improve our knowledge of iGASD. Since the importance of this disease is well known, in 2015 a network was created to investigate iGASD in the paediatric population in the Autonomous Community of Madrid.
In conclusion, our study on iGASD in children found an increase in incidence in the catchment area of our hospital in Madrid. This disease continues to have the potential to be very severe, with a high percentage of affected patients admitted to the PICU and requiring surgery. Given the heterogeneity of the studies published to date, it would be interesting to establish a prospective, multicentre nationwide register of iGASD, to accurately evaluate its epidemiology, risk factors and severity, and to establish the characteristics of the strains isolated in affected children.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank our colleagues Elena María Rincón López and Begoña Santiago García, from the Department of Paediatric Infectious Diseases, for their continuous support and their advice during the study; Carlos Sánchez-Carrillo and Mercedes Marín, from the Department of Microbiology and Infectious Diseases, for their collaboration in providing data on blood cultures and molecular tests and their valuable corrections, and José María Bellón Cano, from the Project Design and Statistical Analysis Support Unit of the Instituto de Investigación Sanitaria del Gregorio Marañón (IISGM), for his help in the statistical analysis.
Please cite this article as: Suárez-Arrabal MC, Sánchez Cámara LA, Navarro Gómez ML, Santos Sebastián MdM, Hernández-Sampelayo T, Cercenado Mansilla E, et al. Enfermedad invasiva por Streptococcus pyogenes: cambios en la incidencia y factores pronósticos. An Pediatr (Barc). 2019;91:286–295.
Previous presentations: Part of this study was presented as an oral communication at the 33rd Annual Meeting of the European Society for Paediatric Infectious Diseases (ESPID); May 12–16, 2015; Leipzig, Germany.