Children are the main source of transmission and reservoir of influenzavirus. It is believed that control of the virus is poorer in the paediatric population, and that therefore children have longer shedding periods compared to other age groups.1 The aim of this study was to analyse the influenza A (H1N1)pdm09 virus kinetics and clearance in hospitalized children and to establish their association with different clinical variables.
We conducted a prospective and observational study in the Departments of Paediatrics and Microbiology of 2 hospitals in Valladolid, Spain, and the National Influenza Centre of Valladolid. The study period ranged from week 40 of 2015 to week 20 of 2016, corresponding to the 2015–2016 flu season. We included inpatients aged less than 14 years with laboratory-confirmed influenza A(H1N1)pdm09 virus infection. For the purpose of testing, we collected throat swab samples in patients aged more than 2 years and samples of nasopharyngeal lavage in younger children. The latter allows a more representative sample to be obtained and could overestimate the viral RNA load (VRL) in this group of patients. To analyse the VRL, we collected samples on the day of admission and days 4, 8 and 12 or until negative, if this occurred before day 12. The variables under study included the duration of symptoms from onset, number of long-term excretors (LTE, defined as patients with periods of viral shedding [VS] greater than 8 days from onset), and number of short-term excretors (STE, defined as patients with VS<8 days). We obtained written informed consent from the legal guardians of every participant.
Influenza A(H1N1)pdm09 virus infection was confirmed by reverse-transcription polymerase chain reaction (RT-PCR) using MAGPIX and NxTAG-RPP reagents (Luminex; Austin, TX, USA). The VRL was measured by quantitative RT-PCR in the influenza-positive samples using a 7500-Fast Real-Time PCR System (Applied Biosystems; Foster City, CA, USA) and LightMix-Kit Influenza A Virus M2 reagents (Roche; Basel, Switzerland). We used the Allplex Respiratory Full Panel (Seegene) reagent kit in every patient, which can detect 19 viral and 7 bacterial targets, including the M2 and H genes of the main endemic strains circulating in Spain. The statistical analysis focused on the comparison of different clinical variables and their relationship with the duration of VS. We used the software SPSS version 20.0 to perform the statistical tests.
We recruited 24 patients (54% male; median age, 17.5 months; age range: 0–120 months) during the study period. Severe asthma and cystic fibrosis were the only comorbidities, detected in two patients (8%), and these were the only patients that received the influenza vaccine. We found the highest VRL in the first sample taken on the day of admission in 87.5% of patients (mean CV1, 7032.9 copies/mL; 95% confidence interval [CI], 1131.2−16 418.5), with a decrease in the second sample (mean CV2, 239.5 copies/mL; 95% CI, 51.4–547.9). All patients tested negative in the third timepoint. Fifty percent of patients (12/24) were LTEs. The mean length of stay was 7.4 days (95% CI, 5.1–9.9) in the LTE group, compared to 5.6 days (95% CI, 3.4–8.0) in the STE group, a difference that was not statistically significant (Student t test P = 0.294) (Table 1).
Demographic and clinical characteristics of the 24 children hospitalized with influenzavirus infection.
| Patient ID | Sex | Age(months) | VRL1(copies/mL) | VRL2(copies/mL) | Excretion | Coinfection | Days from onset | Length of stay (days) | PICU admission | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 17 | 3280 | 14 | LTE | Bacterial(MC) | 1 | 5 | No | AC |
| 2 | F | 16 | 6240 | UD | STE | No | 4 | 9 | No | ACOseltamivir |
| 3 | M | 14 | 246 | 2900 | LTE | Bacterial (SP, HI) | 3 | 5 | No | AC |
| 4 | F | 4 | 89 200 | UD | STE | No | 2 | 2 | No | C |
| 5 | F | 48 | 11 400 | 811 | LTE | No | 21 | 6 | Yes | Not ATB/AV |
| 6 | F | 7 | 489 | 823 | LTE | Bacterial (MC) | 4 | 7 | No | C |
| 7 | F | 48 | 781 | 16 | LTE | No | 1 | 6 | No | C+V |
| 8 | M | 26 | 1330 | 32 | LTE | Viral (B) | 5 | 7 | Yes | AZT |
| 9 | F | 15 | 27 | UD | STE | Viral (A) | 1 | 4 | No | C |
| 10 | F | 0 | 27 | UD | STE | Viral (RSV) | 2 | 13 | Yes | AMP+G |
| 11 | M | 12 | 41 800 | UD | STE | No | 2 | 1 | No | Not ATB/AV |
| 12 | M | 18 | 53 | UD | LTE | Viral (B) | 5 | 1 | No | AC |
| 13 | M | 35 | 17 | UD | STE | No | 1 | 10 | No | AC |
| 14 | M | 18 | 651 | 720 | LTE | No | 8 | 8 | Yes | C+AZT |
| 15 | M | 48 | 7540 | UD | STE | No | 1 | 5 | Yes | Not ATB/AV |
| 16 | M | 0 | 749 | 309 | LTE | Bacterial(MC) | 1 | 10 | No | AC |
| 17 | M | 0 | 267 | 48 | LTE | Bacterial(SA) | 1 | 15 | No | AC |
| 18 | M | 48 | 22 | UD | LTE | No | 7 | 6 | No | Not ATB/AV |
| 19 | M | 120 | 3100 | UD | STE | No | 1 | 5 | No | C Oseltamivir |
| 20 | M | 13 | 86 | UD | STE | Viral(B, RSV) | 7 | 5 | No | AC |
| 21 | M | 84 | 359 | UD | STE | No | 3 | 3 | No | Oseltamivir |
| 22 | F | 108 | 832 | UD | STE | No | 1 | 9 | No | AMP |
| 23 | F | 48 | 252 | 76 | LTE | No | 6 | 16 | Yes | AMP+COseltamivir |
| 24 | F | 1 | 42 | UD | STE | Bacterial(MC) | 1 | 1 | No | AC |
A, adenovirus; AC, amoxicillin-clavulanic acid; AMP, ampicillin; ATB/AV, antibiotic/antiviral; AZT, azithromycin; B, bocavirus; C, cefotaxime; F, female; G, gentamicin; HI, Haemophilus influenzae; LTE, long-term excretor; M, male; MC, Moraxella catarrhalis; PICU, paediatric intensive care unit; RSV, respiratory syncytial virus; SA, Staphylococcus aureus; SP, Streptococcus pneumoniae; STE, short-term excretor; UD, undetectable; V, vancomycin; VRL1, viral RNA load in the first sample; VRL2, viral RNA load in the second sample.
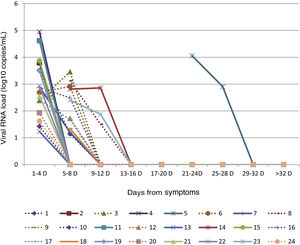
The VRL became undetectable after 9–12 days from onset in 67% (8/12) of LTEs, between 13 and 16 days in 25% (3/12) and after 12 days in 8% (1/12) (Fig. 1). We found viral or bacterial coinfections with influenza in 58.3% (7/12) of LTEs (5 bacterial and 2 viral). Six patients required admission to the paediatric intensive care unit (PICU), 4 of them were LTEs. The 4 LTEs required respiratory support with non-invasive ventilation (NIV), and none required vasoactive drugs. We detected viral or bacterial coinfection in 45.8% of patients (11/24), bacterial in 54.5% (6/11) (involving Moraxella catarrhalis, Streptococcus pneumoniae, Staphylococcus aureus and Haemophilus influenza), and viral in 45.5% (5/11) (involving bocavirus, respiratory syncytial virus and adenovirus). We did not find significant differences in the VRL detected in the first (Student t test P = 0.180) or in the second (Student t test P = 0.059) between the LTE and STE groups. The approach to treatment was similar in both groups: 100% received symptomatic management, 79% received antibiotherapy (Table 1), 54% bronchodilator therapy, 50% required respiratory support (33% in the form of NIV). Only 4 patients that developed neurologic manifestations received oseltamivir, all of them in the STE group.
Data from our study show that half of the paediatric patients hospitalized on account of a laboratory-confirmed infection of influenza A(H1N1)pdm09 virus had a detectable VRL past day 8 from onset and 17% beyond day 12. Patients with longer duration of disease had longer VS periods, although the length of stay was similar in both groups. This could mean that LTEs have poorer control of viral clearance compared to STEs, which would increase the duration of the respiratory illness, but not their required length of stay.
The paediatric population generally has longer VS periods in influenza infections compared to adults,2 which can be attributed to the immaturity of their immune systems, a history of immunosuppression, the need for admission to the PICU or infection with antiviral-resistant influenza strains.3,4 There is also evidence of viral and bacterial coinfections with influenzavirus being common in LTEs, which may increase disease severity and the risk of hospital admission.5 In our cohort, we found that most patients admitted to the PICU or with bacterial coinfections were LTEs. However, we did not find a significant correlation between LTE status and admission to PICU or bacterial coinfection, probably due to the small sample size.
In conclusion, our findings show that half of paediatric inpatients with laboratory-confirmed influenza A(H1N1)pdm09 virus infection are LTEs, and most of the patients that required intensive care or with bacterial coinfection were LTEs. Monitoring VRL changes in hospitalized paediatric patients could help predict the severity of disease and identify the need of additional microbiological tests.
FundingThis study was funded by the Department of Health of Castilla y Leon (project number GRS 1094/A/15).
Please cite this article as: Sánchez N, Matías V, Alcalde C, Rojo S, Sanz I. Influenza A (H1N1)pdm09 viral clearance kinetics in hospitalized children. An Pediatr (Barc). 2021;95:271–274.
Previous presentation: this study was presented as an oral communication at the 67th Congress of the Asociación Española de Pediatría, June 6-8, 2019, Burgos, Spain.