Congenital heart defects (CHDs) are the most frequently diagnosed congenital anomalies in children in Europe. The spectrum of disease ranges from complex malformations associated with a high mortality to benign septal defects. Based on data from the European Surveillance of Congenital Anomalies (EUROCAT) network, the prevalence of CHDs in the absence of genetic abnormalities in Europe in the 2000–2018 period was of 0.58% of all live births.1
The literature on international adoption shows that congenital anomalies are overrepresented in the population of internationally adopted (IA) children, with differences in prevalence based on the country of origin.2 The aim of our study was to establish the prevalence of CHDs in a cohort of IA children and analyse differences based in country of origin.
We conducted a retrospective observational study by reviewing the health records of 439 children adopted from another country by Spanish families in the 2000–2018 period and evaluated on arrival to Spain in a specialised adoption clinic. We collected data on the following: age, sex, country of origin, history of corrected CHD, clinical signs or symptoms that led to the cardiac assessment and type of CHD diagnosed by echocardiography.
Of the 439 children (mean age, 29 months; 50.8% male) 300 were adopted from Russia, 35 from China, 32 from Eastern European countries (28 from Ukraine, 2 from Romania and 2 from Moldavia), 22 from Kazakhstan, 16 from the Indian subcontinent (11 from India, 5 from Nepal), 16 from Latin American countries (7 from Colombia, 4 from Bolivia, 2 each from Brazil and Uruguay and 1 from Ecuador), 10 from countries in South East Asia (7 from Vietnam, 3 from Philippines) and 8 from Ethiopia. Two children adopted from Russia had a history of CHD surgically corrected in the country of origin (tetralogy of Fallot in 1 and patent ductus arteriosus in 1). The only clinical sign that prompted performance of a cardiac assessment was detection of a heart murmur in the evaluation performed on arrival to Spain. This occurred in 54 children (12.3% of the total), of who 47 were from Russia, 4 from Ukraine, 1 from Kazakhstan, 1 from Bolivia and 1 from Ethiopia. The echocardiographic evaluation confirmed the presence of CHDs in 18 children (33.3% of the children with a heart murmur).
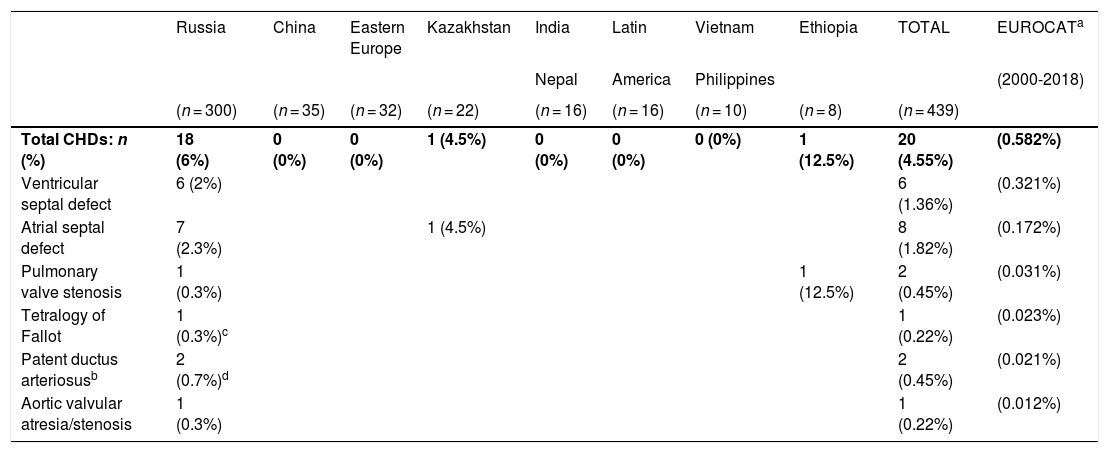
Table 1 presents the frequency distribution and prevalence of the CHDs identified in IA children compared to the prevalence observed by the EUROCAT surveillance network2 in infants born alive without genetic abnormalities in the 2000-2018 period. The prevalence of CHDs in the cohort of IA children was 4.55%, 7.8 times higher compared to the prevalence reported by the EUROCAT. The male-to-female ratio was 1.5. Ninety percent of IA children with CHDs had been adopted from Russia. Applying the definitions of the EUROCAT, 80% of CHDs were minor. The most frequent CHDs were atrial septal defect (ASD, 40% of the total) and ventricular septal defect (VSD, 30% of the total).
Prevalence of congenital heart defects (CHDs) in internationally adopted children and in the EUROCAT study (2000-2018)
| Russia | China | Eastern Europe | Kazakhstan | India | Latin | Vietnam | Ethiopia | TOTAL | EUROCATa | |
|---|---|---|---|---|---|---|---|---|---|---|
| Nepal | America | Philippines | (2000-2018) | |||||||
| (n = 300) | (n = 35) | (n = 32) | (n = 22) | (n = 16) | (n = 16) | (n = 10) | (n = 8) | (n = 439) | ||
| Total CHDs: n (%) | 18 (6%) | 0 (0%) | 0 (0%) | 1 (4.5%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (12.5%) | 20 (4.55%) | (0.582%) |
| Ventricular septal defect | 6 (2%) | 6 (1.36%) | (0.321%) | |||||||
| Atrial septal defect | 7 (2.3%) | 1 (4.5%) | 8 (1.82%) | (0.172%) | ||||||
| Pulmonary valve stenosis | 1 (0.3%) | 1 (12.5%) | 2 (0.45%) | (0.031%) | ||||||
| Tetralogy of Fallot | 1 (0.3%)c | 1 (0.22%) | (0.023%) | |||||||
| Patent ductus arteriosusb | 2 (0.7%)d | 2 (0.45%) | (0.021%) | |||||||
| Aortic valvular atresia/stenosis | 1 (0.3%) | 1 (0.22%) | (0.012%) |
Prevalence (%) of CHDs relative to number of live births, EUROCAT study, 2000 to 2018, all cases included except for genetic disorders. Updated data, including the cohort born in 2018 (as of 29/09/2020). Source: https://eu-rd-platform.jrc.ec.europa.eu/eurocat/eurocat-data/prevalence_en.
It is known that CHDs may result from cytogenetic abnormalities, monogenic variants, environmental factors or, most frequently, have a multifactorial aetiology.3 Increases in maternal exposure to environmental risk factors for CHDs during gestation, such as alcohol, tobacco or drug use, consumption of teratogenic medicines, exposure to organic solvents, viral infection in the first trimester etc, as well as inadequate management of chronic conditions in the mother during gestation (diabetes, obesity, hypertension etc) are among the factors to take into account to explain the high prevalence of CHDs in IA children.2–4
The most salient finding of our study was the high prevalence of CHDs in children adopted from Russia. It is known that the frequency of prenatal alcohol exposure (PAE) is high in children residing in orphanages in Russia who are candidates for international adoption.5 Burd et al.6 reviewed the literature on the prevalence of CHDs associated with foetal alcohol spectrum disorder (FASD) and found the following: in 12 case series of individuals with FASD, the prevalence of septal defects, other specific CHDs or defects of unspecified type ranged from 33% to 100%; in 14 retrospective studies, the prevalence of septal defects was of 21%, the prevalence of other structural defects 6% and the prevalence of unspecified defects 12%; in 2 case-control studies, the odds of CHD ranged from 1.0 in individuals with FASD to 18.0 in individuals with foetal alcohol syndrome; in 1 prospective study, the odds ratio for a child having both CHD and FASD was 1.0.
To establish the role of PAE as a potential underlying cause of CHDs in the IA children cohort, we reviewed preadoption medical evaluations (PMEs) looking for documentation of PAE. We did not find any mention of PAE in the PMEs of children adopted from Latin America, China, India, Nepal, Vietnam, Philippines, Ethiopia, Kazakhstan or Eastern Europe. The PMEs of children adopted from Russia reported a history of PAE in 31% of the total (n = 93) and 33.3% of those with CHDs (n = 6). Unfortunately, as it is well known, the history of PAE is rarely documented in PMEs in China, India, Nepal, Ethiopia and Vietnam, and is frequently omitted from PMEs in Russia, Eastern European countries and Kazakhstan. It is important to be aware that the absence of documentation of maternal chronic diseases or other disorders or conditions associated with social factors in the PME does not rule out their presence.
Based on our findings, it is reasonable to hypothesise that PAE may play an important role as an environmental factor in the aetiology of the CHDs observed in children adopted from Russia.
Please cite this article as: Oliván-Gonzalvo G, Gracia-Balaguer J. Prevalencia de cardiopatías congénitas en niños adoptados internacionalmente. An Pediatr (Barc). 2021;95:274–275.