Neonatal Candida spp. infections are serious events due to their morbidity and mortality, however, epidemiological information is insufficient in developing countries. The objective of this study was to describe the incidence and factors associated with invasive infection by Candida spp. in a Neonatal Intensive Care Unit in Mexico.
MethodsCase-control study nested in a cohort and matched for birth weight. We estimated the incidence of invasive neonatal infection by Candida spp. For the bivariate analysis of the studied factors, McNemar's test was used to contrast hypotheses and multivariate analysis was made with logistic regression.
ResultsThe incidence of infection was 2.27 events/1000 live newborns. The species identified were C. albicans 35.3% (n 30), C. parapsilosis 30.6% (n 26), C. glabrata 31.8% (n 27) and two events with C. lipolytica. The factors associated with a higher risk were mechanical ventilation (OR 3.04, 95% CI 1.13–8.14), systemic antibiotics (OR 7.48, 95% CI 1.30–42.9), number of antimicrobial regimens (OR 2.02, 95% CI 1.01–4.03), and days with total parenteral nutrition (OR 1.14, 95% CI 1.04–1.25) or with venous catheter central (OR 1.11, 95% CI 1.02–1.20). Fluconazole prophylaxis decreased the risk (OR 0.32, 95% CI 0.12−0.84).
ConclusionsInvasive interventions (central catheter, mechanical ventilation, and parenteral nutrition) and the use of antimicrobials increase the risk of neonatal Candida spp. Infection, while prophylactic fluconazole is protective.
Las infecciones neonatales por Candida spp. son eventos graves por su morbimortalidad, sin embargo, en países en vías de desarrollo la información epidemiológica es insuficiente. El objetivo de este estudio fue describir la incidencia y los factores asociados a infección invasiva por Candida spp. en una Unidad de Cuidados Intensivos Neonatales de México.
MétodosEstudio de casos y controles anidado en una cohorte y apareado por el peso al nacer. Se estimó la incidencia de infección neonatal invasiva por Candida spp. y para el análisis bivariado de los factores estudiados se usó prueba de McNemar para contraste de hipótesis y análisis multivariado con regresión logística.
ResultadosLa incidencia de la infección fue de 2,27 eventos/1.000 RN vivos. Las especies identificadas fueron C. albicans 35,3% (n 30), C. parapsilosis 30,6% (n 26), C. glabrata 31,8% (n 27) y dos eventos con C. lipolytica. Los factores asociados a mayor riesgo fueron la ventilación mecánica (OR 3,04, IC 95% 1,13–8,14), los antibióticos sistémicos (OR 7,48, IC 95% 1,30–42,9), el número de esquemas antimicrobianos (OR 2,02, IC 95% 1,01–4,03), y los días con nutrición parenteral total (OR 1,14, IC 95% 1,04–1,25) o con catéter venoso central (OR 1,11, IC 95% 1,02–1,20). La profilaxis con fluconazol disminuyó el riesgo (OR 0,32, IC 95% 0,12–0,84).
ConclusionesLas intervenciones invasivas (catéter central, ventilación mecánica y nutrición parenteral) y el uso de antimicrobianos incrementan el riesgo de infección neonatal por Candida spp., mientras que el fluconazol profiláctico es protector.
Candida species are an important cause of morbidity and mortality in hospitalised newborns,1–3 and candidiasis carries a risk of severe sequelae, including neurologic impairment, visual impairment and/or hearing loss and bronchopulmonary dysplasia.4–6
Candida albicans is the most prevalent species, but others, such as Candida parapsilosis, Candida tropicalis, Candida krusei and Candida glabrata, have been increasing in frequency.2,4 Infection and/or colonization of the skin, digestive tract or airways can be acquired in the birth canal or through contact with the hands of health care staff during the hospital stay. Between 5% and 10% of colonised patients develop invasive disease, and once the microorganisms enter the bloodstream, they can invade tissues such as the nervous central system, kidneys, liver, spleen, heart or retina.4,7
The risk of infection is inversely proportional to gestational age and birth weight, due, among other factors, to the immaturity of the immune system4,8 and the frequent use of invasive techniques for respiratory and haemodynamic support.2,3,6,9 The use of broad-spectrum antibiotics increases the density of yeasts in colonised patients, as it decreases the competition posed by commensal bacteria.3,4 Although microbiological tests are currently available that allow confirmation or ruling out of bacteraemia in the following 72 h, a high percentage of hospitalised newborns are exposed to broad-spectrum antibiotics and for prolonged periods.3,10
In developed countries, the incidence of neonatal candidiasis has decreased by 61% in the past few decades, and the interventions that have facilitated this change were the use of fluconazole prophylaxis, decreased prescription of broad-spectrum antibiotics and improved protocols for central catheter insertion and handling.3,4 In developing countries, there is a dearth of information, and the epidemiological data are not consistent. The aim of our study was to measure the incidence of neonatal infection by Candida spp in a neonatal intensive care unit (NICU) in western Mexico and analyse the factors associated with these infections.
MethodsWe conducted a nested case-control study in a cohort managed at the Hospital Civil de Guadalajara Dr. Juan I. Menchaca (HCGJIM) in Ciudad de Guadalajara, Mexico. This hospital provides services to an open low-income population. The department of neonatology comprises an 18-bed NICU and a 57-bed intermediate care nursery.
We selected patients from a cohort documented from January 1, 2015 to 31 December 2019, which contains demographic and clinical information for every infant born in the hospital (date, sex, mode of delivery, 5-minute Apgar score, birth weight and gestational age at birth).
We identified neonatal cases of nosocomial invasive infection by Candida spp in the electronic records of the microbiology department of bacterial and fungal strain typing tests (Vitek® 2 system), from which we obtained the names and medical record numbers of the patients. For each case, we selected as a control the hospitalised newborn that had been born immediately after with a similar birth weight (±100 g). We did not include patients from other hospitals or outside the established study period.
For all cases and controls, we reviewed the health records to collect data on the variables under study: mechanical ventilation, central venous catheter (CVC), total parenteral nutrition (TPN), bacteraemia, postnatal steroid therapy, use of antacids, antibiotherapy, antifungal prophylaxis and comorbidities. In the case group, we only considered these variables as present if they were implemented before the diagnosis of invasive yeast infection. In the case group, we also collected information on the age at diagnosis of fungal infection, the isolated species, meningeal, cardiac or ophthalmic involvement and whether the infection was persistent and/or recurrent. We estimated the necessary sample size to identify an OR of 4 with an alpha level of 0.05 and a power of 80% using the formula recommended for matched case-control studies.
In the HCGJIM, samples for microbiological testing are obtained following the protocol for diagnosis of neonatal sepsis of the Department of Health.11 Blood and cerebrospinal fluid (CSF) samples are obtained using aseptic technique and inoculated in BacT/ALERT PF Pediatric FAN® bottles. Microbial growth is monitored with the automated Bact/ALERT®3D system for 7 days. Samples in which microbial growth is detected are seeded in blood and MacConkey agar, and, in the case of detection of yeasts in the Gram stain, also in Sabouraud agar. The identification of the microbial genus and species and antimicrobial susceptibility testing were performed with the Vitek® 2 automated system. The microorganisms were inoculated in dehydrated panels and incubated at 35 °C for 16–24 h, in adherence with the standards established by the Clinical and Laboratory Standards Institute.12
DefinitionsInvasive infection by Candida spp: clinical manifestations of infection (fever, hypothermia, tachycardia, bradycardia, polypnea, decreased activity, decreased appetite, vomiting and/or diarrhoea) and isolation of any Candida species from normally sterile sites: blood, CSF, urine, peritoneal fluid, pleural fluid, joint fluid.
Persistent infection by Candida spp: culture positive for Candida spp after more than 5 days of antifungal treatment to which susceptibility testing showed in vitro sensitivity.
Recurrent infection: microbiologically confirmed infection by Candida spp in a patient with a history of a previous infection by Candida spp that was treated correctly at least 2 weeks after the negative culture results.
Statistical analysisFor the total cohort of infants born during the study period, we estimated the overall and annual incidence of episodes of invasive infection by Candida spp per 1000 births with the corresponding 95% confidence intervals (CIs). We compared the incidence in different birth weight and gestational age subgroups and estimated relative risks (RRs) with their 95% CIs using the subsets of newborns with birth weights of 2500 g or less and born at a gestational age of 37 or more weeks as reference groups.
In the matched pairs of cases and controls, we calculated absolute frequencies and percentages for qualitative variables and the mean and standard deviation (SD) for quantitative variables if the data were normally distributed or, otherwise, the median and interquartile range (IQR). For hypothesis testing, we used the chi square test to compare proportions and the Student t test or Mann-Whitney U test to compare means or medians, respectively. We conducted a bivariate analysis, calculating odds ratios (ORs) for the matched case-control pairs and using the McNemar test for hypothesis testing. Variables in which comparisons yielded a P value of 0.02 or less were included in the multivariate logistic regression analysis. The statistical analysis was performed with the statistical packages IBM SPSS® Statistics version 20 and OpenEpi (http://openepi.com/Menu/OEMenu.htm). The study was approved by the Research and Ethics Committees of the HCGJIM under file number 0418/20.
ResultsDuring the period under study, the hospital managed 37 462 deliveries, with a median gestational age of 39.0 weeks (maximum, 42.0; minimum, 22.0; IQR, 3.0) and a median birth weight of 3045 g (maximum, 5543; minimum, 410; IQR, 660.0). A total of 85 episodes of infection by Candida spp were diagnosed, corresponding to an estimated overall incidence of 2.27 infections/1000 births (95% CI, 1.82–2.79). Broken down by year, we found the lowest incidence in 2016 (0.85 infections/1000 births; 95% CI, 0.39−1.62) and the highest in 2018 (6.0 infections/1000 births; 95% CI, 4.31−8.14).
When we compared the patients with infection by Candida spp and the rest of the newborns during the study period, we found significant differences in weight (1928.6 vs 2993.17 g; P < .001) and gestational age (33.33 vs. 38.32 weeks; P < .001). The incidence of infection increased significantly with decreasing gestational age and decreasing birth weight (Table 1).
Risk of neonatal infection by Candida species based on gestational age, birth weight and year of diagnosis.
| Events | Patients | Cases/1000 births (95% CI) | Relative risk (95% CI) | P | |
|---|---|---|---|---|---|
| Gestational age | |||||
| ≥37 weeks | 16 | 32 350 | 0.49 (0.29−0.79) | 1 | |
| 34−36.9 weeks | 26 | 3628 | 7.17 (4.78−10.34) | 14.59 (7.82−27.22) | <.001 |
| 32−33.9 weeks | 14 | 729 | 19.20 (10.98−31.26) | 39.57 (19.24−81.38) | <.001 |
| <32 weeks | 29 | 755 | 38.41 (26.37−54.0) | 80.72 (43.65−149.28) | <.001 |
| Birth weight | |||||
| ≥2500 g | 20 | 31 505 | 0.63 (0.40−0.96) | 1 | |
| 1500−2499 g | 31 | 4998 | 6.20 (4.29−8.69) | 9.82 (5.60−17.25) | <.001 |
| <1500 g | 34 | 959 | 34.45 (25.07−48.64) | 57.86 (33.18−100.9) | <.001 |
| Year of diagnosis | |||||
| 2015 | 12 | 9829 | 1.22 (0.66−2.08) | 1 | |
| 2016 | 8 | 9405 | 0.85 (0.39−1.62) | 0.70 (0.28−1.70) | .43 |
| 2017 | 20 | 8172 | 2.45 (1.53−3.70) | 2.00 (0.98−4.10) | .051 |
| 2018 | 38 | 6333 | 6.00 (4.31−8.14) | 4.91 (2.57−9.40) | <.001 |
| 2019 | 7 | 3723 | 1.88 (0.83−3.72) | 1.54 (0.6−3.92) | .36 |
CI, confidence interval.
In infected newborns, the median age at diagnosis was 14.0 days (maximum, 159; minimum, 3; IQR, 15). In 35.3% (n = 30), the infection was caused by Candida albicans, and in the rest of cases, by Candida parapsilosis (30.6%; n = 26), Candida glabrata (31.8%; n = 27) and Candidad lipolytica (n = 2). In 98.8% (n = 84), yeasts were isolated in the blood culture, in 20% (n = 17) in CSF and blood cultures. Two patients developed endocarditis, 2 endophthalmitis, and in 1, microbial isolation only occurred in the peritoneal fluid. Twenty-eight percent (7/25) of the patients in whom a urine sample was obtained had a fungal urinary tract infection. Thirty-nine patients (45.9%) had persistent infection after 5 days of antifungal treatment, and 5 had recurrent infections.
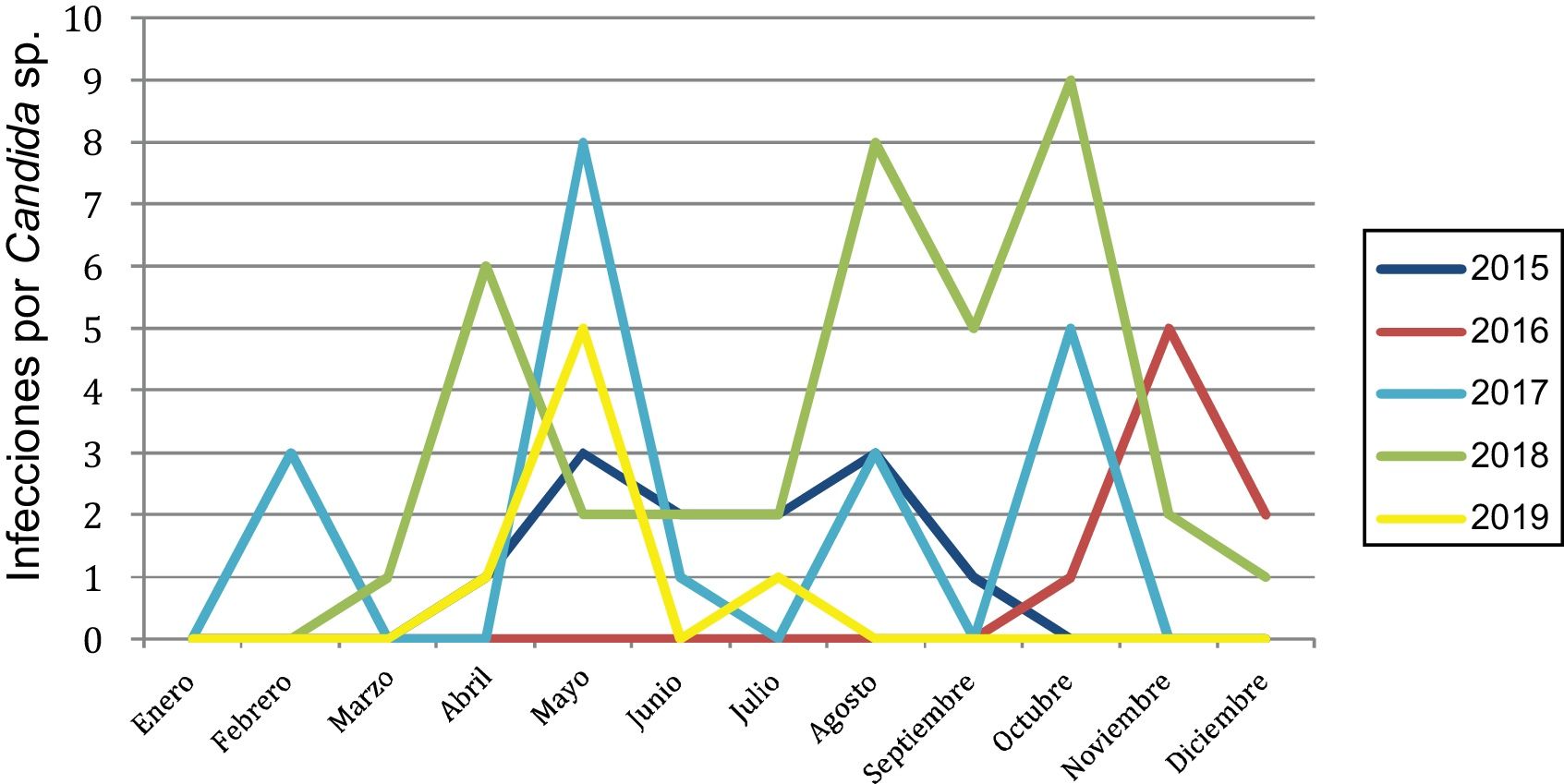
Most infections (83.5%; n = 71) occurred between April and October, the warmest months of the year. This was a recurrent trend, except in 2016, when all the infections occurred in the last trimester of the year (Fig. 1).
The case and control groups were similar in terms of the 5-minute Apgar score, sex, mode of delivery, birth weight and gestational age. In the case group, the use of CVCs, mechanical ventilation, TPN, systemic antibiotherapy and antacids and the presence of abdominal comorbidities were significantly greater. The mean length of stay (56.0 vs 10.0 days; P < .001) and mortality (32.9% vs 18.8%; P = .02) were greater in the case group (Table 2).
Comparison of newborns with infection by Candida species and matched controls.
| Cases | Controls | P* | ||
|---|---|---|---|---|
| n = 85 | n = 85 | |||
| Gestational age | Median, weeks | 34.2 | 33.6 | .65 |
| Birth weight | Median, g | 1720 | 1740 | .96 |
| 5-min Apgar score | Median, puntos | 9.0 | 9.0 | .58 |
| Male sex | Percentage | 52.9 | 54.1 | .87 |
| Caesarean delivery | Percentage | 72.9 | 69.4 | .61 |
| Central vascular accessa | Percentage | 100 | 56.5 | <.001 |
| Days of central vascular access | Median, days | 13.0 | 3.0 | <.001 |
| Mechanical ventilation | Percentage | 88.2 | 41.2 | <.001 |
| Days of mechanical ventilation | Median, days | 8.0 | 0.0 | <.001 |
| Total parenteral nutrition | Percentage | 100 | 49.4 | <.001 |
| Days of parenteral nutrition | Median, days | 13.0 | 0.0 | <.001 |
| Systemic antibiotics | Percentage | 97.6 | 54.1 | <.001 |
| Treatment with meropenem or cefotaxime | Percentage | 54.1 | 14.1 | <.001 |
| Treatment with vancomycin | Percentage | 74.1 | 30.6 | <.001 |
| Total antibiotic courses | Median, courses | 2.0 | 1.0 | <.001 |
| Antifungal prophylaxis with fluconazole | Percentage | 37.6 | 29.4 | .26 |
| Systemic steroids | Percentage | 27.1 | 15.3 | .06 |
| Antacids | Percentage | 64.7 | 25.9 | <.001 |
| Abdominal comorbidityb | Percentage | 35.3 | 8.2 | <.001 |
| Enterocolitis | Percentage | 8.2 | 4.7 | .35 |
| Bacteraemia | Percentage | 43.5 | 15.3 | <.001 |
| Length of stay | Median, days | 56.0 | 10.0 | <.001 |
| Deaths | Percentage | 32.9 | 18.8 | .03 |
P values of less than .05 were considered statistically significant.
There were intestinal comorbidities in 35.3% of the cases and 8.2% of the controls, and in both groups, the most frequent ones were gastroschisis and necrotising enterocolitis.
We found that newborns with invasive fungal infection were more likely to have bacterial bloodstream infections (43.5% vs 15.3%; P < .001), and when we compared subgroups based on the isolated pathogens, we found that bacteraemia by Klebsiella pneumoniae was more frequent in newborns with fungal infection (27.0% vs 14.1%; P .04) (Table 3).
Bacteria isolated from the bloodstream in cases and controls.
| Group or species | Cases | Controls | P < .001 |
|---|---|---|---|
| 37/85a | 13/85b | ||
| Klebsiella pneumoniae | 23 | 12 | .04 |
| Escherichia coli | 5 | 1 | .09 |
| Enterobacter cloacae | 3 | 0 | .12 |
| Coagulase-negative Staphylococcus | 3 | 0 | .12 |
| Pseudomonas aeruginosa | 2 | 0 | .24 |
| Sphingomonas paucimobilis | 1 | 0 | .5 |
| Acinetobacter baumannii | 1 | 1 | 1 |
| Enterococcus faecalis | 1 | 0 | .5 |
| Haemophilus influenzae | 1 | 0 | .5 |
| Leclercia adecarboxylata | 0 | 1 | .5 |
In the case groups, bloodstream bacterial infections occurred prior to detection of fungemia.
The use of systemic antibiotics was more frequent in newborns with infection by Candida spp (97.6 vs 54.1%; P < .001); in both groups the initial course of antibiotherapy consisted of ampicillin/gentamycin in the first 3 days of life followed by vancomycin/amikacin after day 3 post birth. More than one course was administered in 70.6% of cases and 28.6% of controls.
In the bivariate analysis of matched cases and controls, we found that the factors associated with invasive fungal infection were the use of invasive techniques such as central vascular catheterization and mechanical ventilation. Other associated factors were TPN, antacid treatment and antibiotic treatment, especially with broad-spectrum antibiotics such as cephalosporins, carbapenems and vancomycin, and abdominal comorbidities and bacteraemia, chiefly caused by enterobacteria (Table 4).
Bivariate analysis of the studied factors and invasive infection by Candida species.
| Odds ratio (95% CI) | P* | |
|---|---|---|
| Matched case-control pairs n = 85 | ||
| Male sex | 0.95 (0.51−1.76) | 1.0 |
| Caesarean delivery | 1.2 (0.60−2.38) | .73 |
| Central cathetera | 75 (4.6−1 221) | <.001 |
| Mechanical ventilation | 41 (5.64−298) | <.001 |
| Total parenteral nutrition | 87 (5.36−1 413) | <.001 |
| Systemic antibiotherapy | 19.5 (4.70−80.7) | <.001 |
| Regimen with meropenem or cefotaxime | 18.0 (4.33−74.75) | <.001 |
| Regimen with vancomycin | 7.17 (3.05−16.8) | <.001 |
| Antifungal prophylaxis with fluconazole | 2.17 (0.82−5.7) | .11 |
| Systemic steroids | 2.43 (1.01−5.86) | .06 |
| Antacids | 7.6 (3.0−19.3) | <.001 |
| Abdominal comorbidity | 4.8 (2.0−11.6) | <.001 |
| Enterocolitis | 1.75 (0.51−5.98) | .55 |
| Bacteraemia | 5 (2.08−12.01) | <.001 |
| Bacteraemia caused by enterobacteria | 3.83 (1.56−9.41) | .002 |
| Bacteraemia caused by Klebsiella pneumoniae | 2.83 (1.12−7.19) | .03 |
| Deaths | 2.7 (1.14−6.46) | .02 |
CI, confidence interval.
Variables with P values ≤.2 were included in the multivariate analysis.
Variables with P values of .2 or less were included in the multivariate logistic regression analysis. We started with a full model including all variables that met that criterion, with stepwise elimination of variables based on statistical significance or their effect on other variables in the model. Table 5 presents the risk factors identified in the multivariate analysis.
Multivariate analysis (logistic regression) of the factors associated with neonatal invasive infection by Candida spp.
| Odds ratio (95% CI) | P | |
|---|---|---|
| Mechanical ventilation | 3.04 (1.13−8.14) | .03 |
| Systemic antibiotics | 7.48 (1.30−42.9) | .02 |
| Number of antimicrobial courses | 2.02 (1.01−4.03) | .04 |
| Days of total parenteral nutrition | 1.14 (1.04−1.25) | .003 |
| Days of central venous catheter | 1.11 (1.02−1.20) | .01 |
| Antifungal prophylaxis with fluconazole | 0.32 (0.12−0.84) | .02 |
CI, confidence interval.
When we compared newborns with infection by C albicans with those with infection by non-albicans spp we only found differences in the use of mechanical ventilation, which was more frequent in the former (100% vs 81.8%; P = .02).
DiscussionIn our study, we found an estimated incidence of neonatal infection by Candida spp of 2.27 cases per 1000 births, RN, with a significantly greater incidence in those born preterm and/or with birth weights of less than 2500 g. In Latin American hospitals, the reported average incidence of candidemia in children 0.81 cases per 1000 hospital admissions, and newborns are the predominant age group (29%).13
The factors independently associated with an increased risk of neonatal infection by Candida spp were mechanical ventilation, systemic antibiotherapy, the number of antibiotic courses and the days of CVC or TPN. Santolaya et al. also found this association of candidemia with TPN and mechanical ventilation in newborns and children.13 In a study by Ting et al., the factors associated with neonatal fungal infections were a 5-minute Apgar score of less than 7 and postnatal administration of steroids,6 but we did not observe these associations in our study.
Different studies have reported a high proportion of broad-spectrum antibiotic prescription preceding neonatal infection by Candida spp.1,5,10,14 Antibiotics alter the microbiota of the digestive and respiratory tracts, leading to a predominance of colonising yeasts. In our study, we found that the use of any type of systemic antimicrobials increased the risk of infection, and that this increase was greater with the use of broad-spectrum antibiotics such as third-generation cephalosporins, carbapenems or vancomycin.
In our patients at the HGGJIM, the frequent prescription of antibiotics was promoted by a high incidence of bacteraemia (29.4%, 50/170), chiefly nosocomial infections (84.7%) in which enterobacteria were the predominant causative agents. Similarly, Caparó Ingram et al. found that bloodstream infections, mechanical ventilation and TPN were factors associated with neonatal candidemia.15
While the characteristics of newborns, such as the immaturity of the immune system, may facilitate the development of bacterial infections, it is important that NICUs implement “care bundles” in the management of central vascular access lines and ventilatory support, in addition to antimicrobial stewardship and surveillance programmes.16–18
In newborns, fungal infections may result from translocation of pathogens from the gastrointestinal tract, and there is evidence that different conditions, such as fasting, the presence of necrotising enterocolitis or abdominal surgery increase the risk of infection.6,15 In our study, the bivariate analysis showed that gastrointestinal comorbidities were associated with a higher probability of invasive fungal infection, but this variable was not found to be independently associated in the multivariate analysis. When we compared patients with and without gastrointestinal comorbidities, we found that in the former group, a higher proportion received systemic antibiotherapy (97.3% vs 69.9%; P = .001) and were managed with mechanical ventilation (89.2% vs 57.9%; P < .001); we also found a greater median duration of central vascular catheterization (15 vs 9 days; P < .001) and TPN (14 vs 6 days; P < .001).
Similar to the findings of multicentre studies conducted in Latin America and Europe,1,15,19 the most prevalent Candida spp in patients managed at the HCGJIM were C albicans and C parapsilosis. We did not find differences in mortality or the associated factors when comparing patients with infection by C albicans versus non-albicans species, except for mechanical ventilation, which was more frequent in the former. Ahangarkani et al. found a higher mortality in children infected by non-albicans Candida spp.20
The ability of Candida spp to form biofilms after colonising devices used in invasive procedures hinders antifungal treatment and increases the risk of persistent or recurrent infection.21 In our patients, we identified persistent infections in 45.9% and recurrent infections in 5.8%. We found that newborns with meningitis caused by Candida were at higher risk of persistent infection (70.6% vs 41.2%; P = .03) and the 4 newborns with endocarditis or endophthalmitis also had persistent infections.
In our study, we found that each year, infections by Candida spp were most frequent in the warm months despite adequate temperature control in the NICU setting. This pattern may be facilitated by the risk factors discussed above, and the environmental temperature may have an impact on the intestinal microbiota of newborns or their caregivers,22,23 although it is not certain that such an effect is actually at play.
In NICUs with an incidence of candidemia greater than 5%, prophylaxis is recommended in newborns with birth weights of less than 1500 g, while its use in units with a lower incidence could correspond to a greater number needed to treat (NNT), which in the literature ranges between 9 and 100 newborns.24–26 In our study, we found that prophylaxis with fluconazole reduced the risk of infection by 68%, and we estimated a NNT of 4.3 in the group with birth weights of less than 1500 g, 23.8 in the group with birth weights of 1500–2499 g and 233 in the group with birth weights of 2500 g and greater.
While prophylaxis mainly benefits newborns with birth weights of less than 1500 g, we found that at the HCGJIM 60% of patients with infections by Candida spp had birthweights of 1500 g or greater, and therefore we propose individualised assessment of the potential effects of preventive or therapeutic strategies in patients with multiple risk factors.
In the HCGJIM, the analysis of fungal infections during the study period allowed the reinforcement of relevant interventions: 1) prescription of antifungal prophylaxis in high-risk newborns, 2) restriction of systemic antibiotherapy to patients meeting specific clinical and laboratory criteria, 3) preferential prescription of amphotericin B deoxycholate over lipid-associated preparations and 4) proactive assessment of dissemination of infection to different organs in patients with bloodstream infections by Candida spp.
Different studies have reported a mortality of 20%–40% for neonatal infection by Candida spp.1,3,4 In our study, the mortality in newborns with yeast infection was 32.9% and increased significantly in those with birth weights of less than 1500 g (55.9 vs 17.6%; P < .001) or that required mechanical ventilation (37.3 vs 0%; P = .02).
The limitations of this study are the retrospective collection of data, which could have led to recall bias, and the limited number of case-control pairs for the subgroup analysis, which hindered the identification of risk factors with a weaker association. While the matching of cases and controls by birth weight could favour the detection of differences attributable to this variable, the bivariate analysis found that the groups were similar in terms of gestational age, Apgar scores and sex.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Lona-Reyes JC, Gómez-Ruiz LM, Cordero-Zamora A, Cortés-González SI, Quiles-Corona M, Pérez-Ramírez RO, et al. Incidencia y factores asociados a candidiasis invasiva en una unidad de cuidados intensivos neonatales de México. An Pediatr (Barc). 2022;97:79–86.