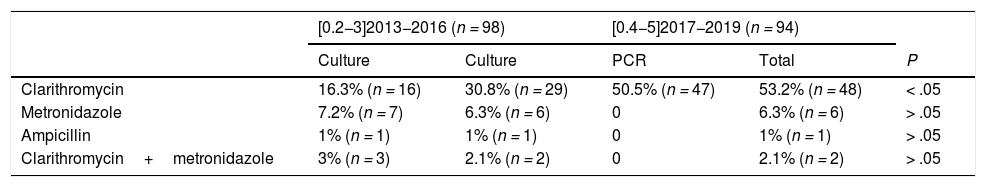Helicobacter pylori infection affects more than 50% of the world population. Increased antibiotic resistance is the main cause of treatment failure. The main objective was to analyze the eradication success after the application of the new ESPGHAN treatment recommendations and the introduction of PCR as a direct diagnosis technique, describe the evolution of the local pattern of antibiotic resistance, and assess the cost-effectiveness of PCR application, isolated or in conjunction with culture as a diagnostic strategy.
Patients and methodsRetrospective descriptive study of all microbiological isolates of Helicobacter pylori in 2013–2019 in our center, by comparing the percentage of resistance and eradication success between the periods 2013−2016 and 2017−2019. Cost-effectiveness study of direct diagnostic tests, comparing 3 different options: culture and PCR; only culture; PCR only.
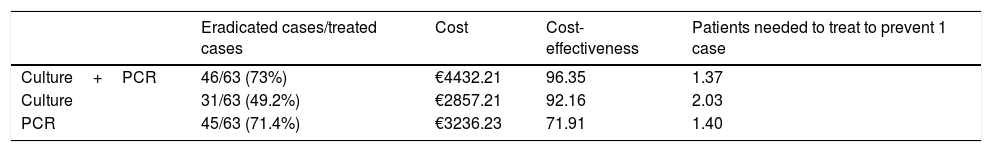
Results192 patients were included, 98 were detected by culture (2013−2016) and 94 by culture and/or PCR (2017−2019). Antibiotic treatment was established in 153 patients, 90 in the first period (2011 ESPGHAN guidelines: eradication percentage 62.2%), 63 in the second (2017 ESPGHAN guidelines: eradication percentage: 73%). An increase in resistance to clarithromycin was observed, going from 16.3% (n=16) in the first period, to 53.2% (n=48) in 2017−2019 (98% detected by PCR, 60% by culture). There were no differences in the rest of antibiotic resistances. The isolated PCR application presented a cost-effectiveness analysis ratio (CEAR) of 71.91, compared to 92.16 for the culture and 96.35 for the culture and PCR combined.
ConclusionsThe application of the ESPGHAN 2017 guidelines achieved greater eradication success, although less than that observed in previous publications, without reaching the target of at least 90%. An increase in resistance to macrolides was observed, without being able to discriminate whether it is a real increase or a greater diagnostic sensitivity of molecular techniques, with the isolated request for PCR being the most cost-effective strategy.
La infección por Helicobacter pylori afecta a más del 50% de la población mundial. El aumento en las resistencias antibióticas es la principal causa del fracaso del tratamiento. El objetivo principal fue analizar el éxito erradicador tras la aplicación de las nuevas recomendaciones de tratamiento ESPGHAN e introducción de la PCR como técnica de diagnóstico directo, describir la evolución del patrón local de resistencias antibióticas y valorar el coste-efectividad de la aplicación de la PCR aislada o en conjunto con el cultivo como estrategia diagnóstica.
Pacientes y métodosEstudio descriptivo retrospectivo del total de aislamientos microbiológicos de Helicobacter pylori entre 2013–2019 en nuestro centro, mediante comparación del porcentaje de resistencias y éxito erradicador entre los periodos 2013−2016 y 2017−2019. Estudio de coste-efectividad de las pruebas de diagnóstico directo, comparando 3 opciones distintas: cultivo y PCR; solo cultivo; solo PCR.
ResultadosSe incluyeron 192 pacientes, 98 fueron detectados por cultivo (2013−2016) y 94 por cultivo y/o PCR (2017−2019). Se instauró tratamiento antibiótico en 153 pacientes, 90 en el primer periodo (pautas ESPGHAN 2011: porcentaje erradicación 62.2%), 63 en el segundo (pautas ESPGHAN 2017: porcentaje erradicación: 73%). Se observó un aumento en las resistencias a claritromicina, pasando de un 16.3% (n=16) en el primer periodo, a un 53.2% (n=48) entre 2017−2019 (98% detectadas por PCR, 60% por cultivo). No hubo diferencias en el resto de resistencias antibióticas. La solicitud aislada de la PCR presentó un ratio de análisis de coste-efectividad (CEAR) de 71.91, en comparación con un 92,16 del cultivo y un 96.35 del cultivo y la PCR de forma conjunta.
ConclusionesLa aplicación de las pautas ESPGHAN 2017 consiguió un mayor éxito de erradicación, aunque menor que lo observado en publicaciones previas, sin llegar al objetivo marcado de al menos un 90%. Se observó un incremento en las resistencias a macrólidos, sin poder discriminar si se trata de un aumento real o de una mayor sensibilidad diagnóstica de las técnicas moleculares, siendo la solicitud aislada de la PCR la estrategia más coste efectiva.
More than half of the global population has been colonised by Helicobacter pylori, with a total of 4.4 billion infected individuals worldwide.1,2 In the healthy paediatric population, the seroprevalence is nearly 33% overall, and as high as 40% in developing countries.3 The infection is usually asymptomatic, although up to 13% of affected individuals may experience complications in the form of peptic ulcers and,4,5 in exceptional cases, gastric cancer or mucosa-associated lymphoid tissue (MALT) lymphoma.6 In 2017, the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) published the latest recommendations for treatment of H. pylori infection.7
The prevalence of drug-resistant H. pylori strains has increased in recent decades. Based on different studies, the prevalence of resistance to clarithromycin in Europe ranges between 17.5% and 49%,4 with the highest prevalence found in southern countries such as Spain or Portugal (32%–49%).8,9 On the other hand, the prevalence of metronidazole in Europe and the United States ranges between 20% and 40%, while the resistance to ampicillin does not exceed 2.5%.10,11 Some of the possible causes of this increase include the massive use of antibiotics in the general population12,13 and the prescription of empiric antibiotherapy not guided by antibiotic susceptibility test results (“test and treat” strategy).14
Culture of a gastric mucosa biopsy specimen is the gold standard for diagnosis, with a sensitivity of 50%–90% and a specificity of nearly 100%, which has led to the detection of resistance to 5 different antibacterial groups.15,16 Molecular techniques, such as polymerase chain reaction (PCR), detect a greater number of cases of drug resistance due to their greater sensitivity and specificity (>95%), although currently they only apply to macrolides and fluoroquinolones.17–19
ObjectivesThe primary objective of our study was to assess the rate of successful eradication after the implementation of the updated ESPGHAN treatment recommendations and the introduction of diagnosis directly by PCR since 20177 compared to the outcomes obtained with the previous recommendations from 201120 and the exclusive use of conventional culture for diagnosis.
The secondary objectives were to describe local trends in antibiotic resistance between 2013 and 2019 and to assess the cost-effectiveness of the use of PCR in isolation or combined with culture for diagnosis.
Sample and methodsWe conducted a retrospective and descriptive study of all the H. pylori cases identified through testing of gastric mucosa specimens by culture or PCR in patients aged 16 years naïve to treatment between 2013 and 2019. We assessed 2 periods (2013−2016 and 2017−2019) based on the introduction of PCR as a diagnostic method17,18 and the publication in 2017 of the updated treatment recommendations of the ESPHGAN.7
We compared the proportion of successful eradication (by per protocol and intention to treat analysis) and the proportion of cases resistant to clarithromycin, metronidazole and ampicillin in each period based on the application of the recommendations published in 201120 and 2017.7 We made comparisons with the chi square test for independent samples and performed the statistical analysis with the software SPSS version 15. Patients did not undergo any interventions outside of the established treatment protocols, and we did not collect data on any other aspects of the patients’ care.
We performed a cost-effectiveness analysis for the second group of patients (2017−2019) to assess the success of eradication therapy based on the use of each of the 3 possible diagnostic approaches. We created mathematical models to simulate real-world practice in our hospital (ordering both culture and PCR in every patient) and 2 hypothetical scenarios (ordering culture only or PCR only). The outcome of interest was the proportion of eradication achieved with each of the options. We generated 3 decision trees and calculated the cost of the direct approach of diagnostic testing (cost of culture: є1 per plate, є24.63 per antibiotic susceptibility test; PCR cost: є25) to obtain the cost effectiveness ratio (CER) (total cost per case of successful eradication).
ResultsThe study included a total of 192 patients, corresponding to 98 cases detected by culture (2013−2016) and 94 by culture or PCR (2017−2019).
Overall, 153 of the patients received antibiotherapy, 90 in the first period (ESPGHAN 2011 guidelines) and 63 in the second period (ESPGHAN 2017 guidelines). Losses to follow-up amounted to 7.8% of patients in the first period (n=7) and 14.3% in the second period (n=10). The proportion of successful eradication was 62.2% (n=56) in the 2013−2016 period compared to 73% (n=45) in the 2017−2019 period, a difference that was statistically significant (P<.05) in the per-protocol analysis but not in the intention-to-treat analysis.
Table 1 presents the results of antibiotic susceptibility testing. We found statistically significant differences in the proportion of resistance to clarithromycin between the 2 periods (16.3% [n=16] in 2013−2016 vs 53.2% [n=48] in 2017−2019; P<.05). Of all cases of clarithromycin resistance in the second period, 98% (n=47) were detected by PCR and 60% (n=29) by culture. We did not find any significant differences in the resistance to other antibiotics.
Helicobacter pylori drug resistance trends.
| [0.2−3]2013−2016 (n = 98) | [0.4−5]2017−2019 (n = 94) | ||||
|---|---|---|---|---|---|
| Culture | Culture | PCR | Total | P | |
| Clarithromycin | 16.3% (n = 16) | 30.8% (n = 29) | 50.5% (n = 47) | 53.2% (n = 48) | < .05 |
| Metronidazole | 7.2% (n = 7) | 6.3% (n = 6) | 0 | 6.3% (n = 6) | > .05 |
| Ampicillin | 1% (n = 1) | 1% (n = 1) | 0 | 1% (n = 1) | > .05 |
| Clarithromycin+metronidazole | 3% (n = 3) | 2.1% (n = 2) | 0 | 2.1% (n = 2) | > .05 |
For the first period (2013−2016), the table shows the cases of resistance identified by culture. For the second period (2017−2019), it shows the cases identified separately by culture and by PCR testing, and the cumulative cases identified with both methods.
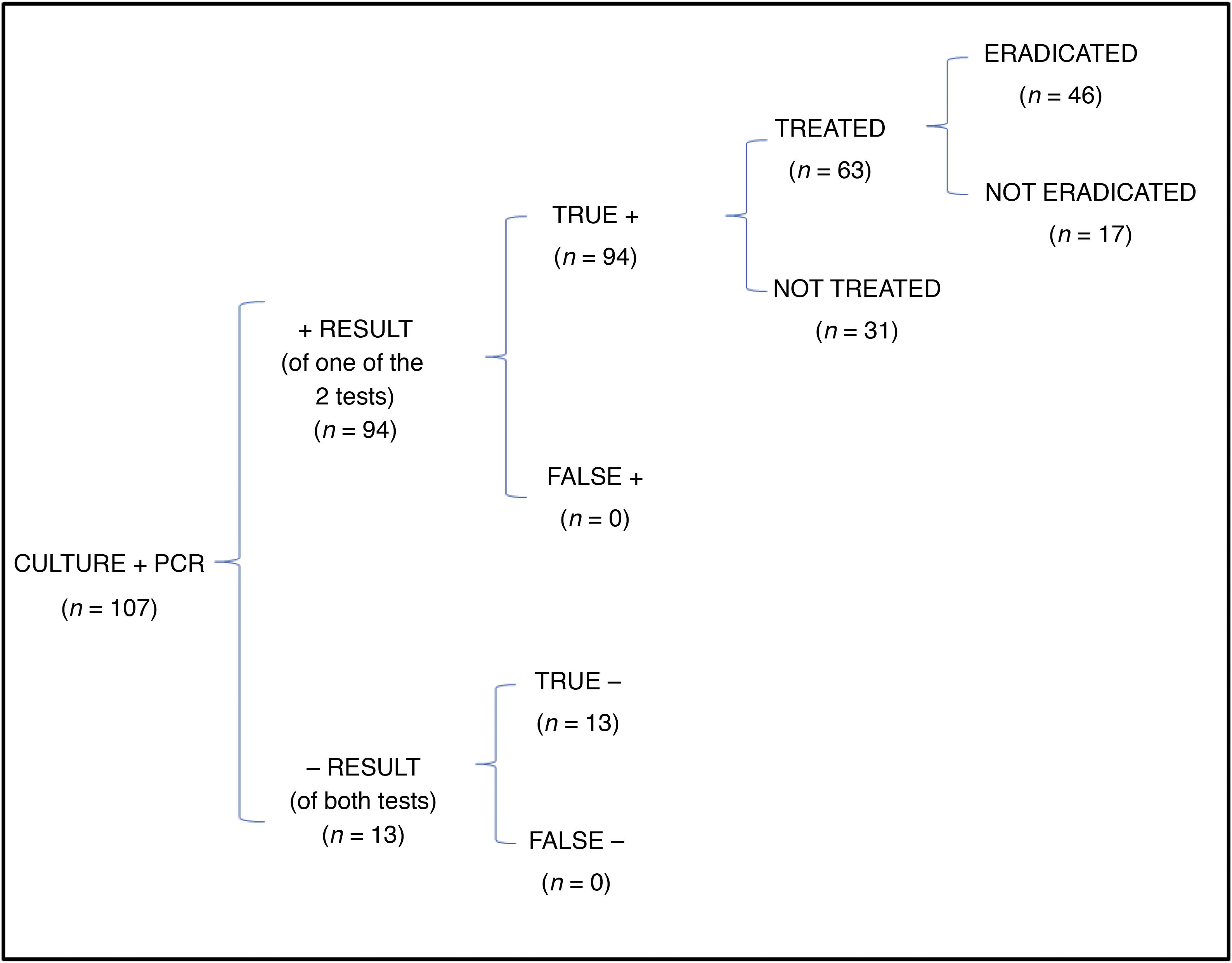
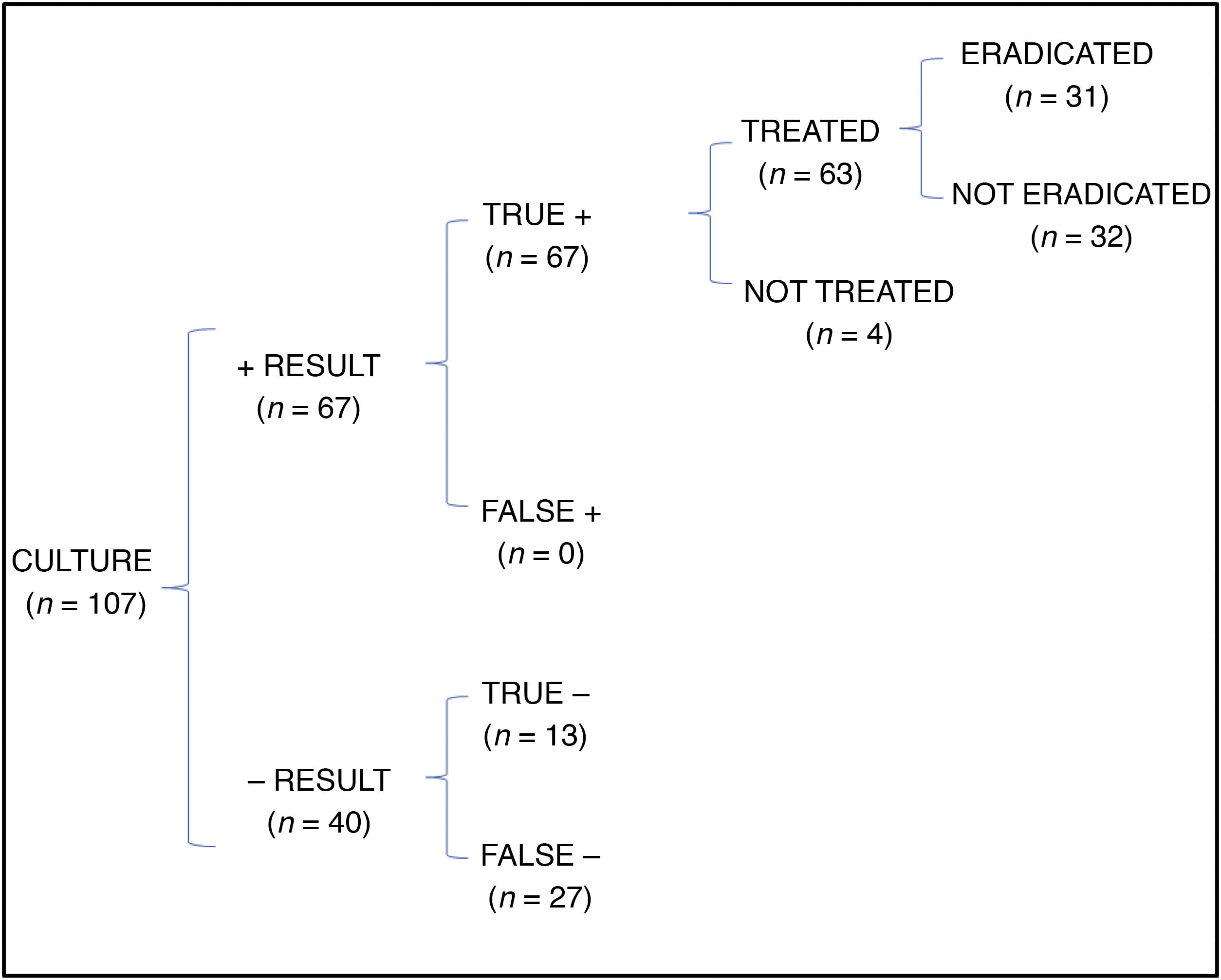
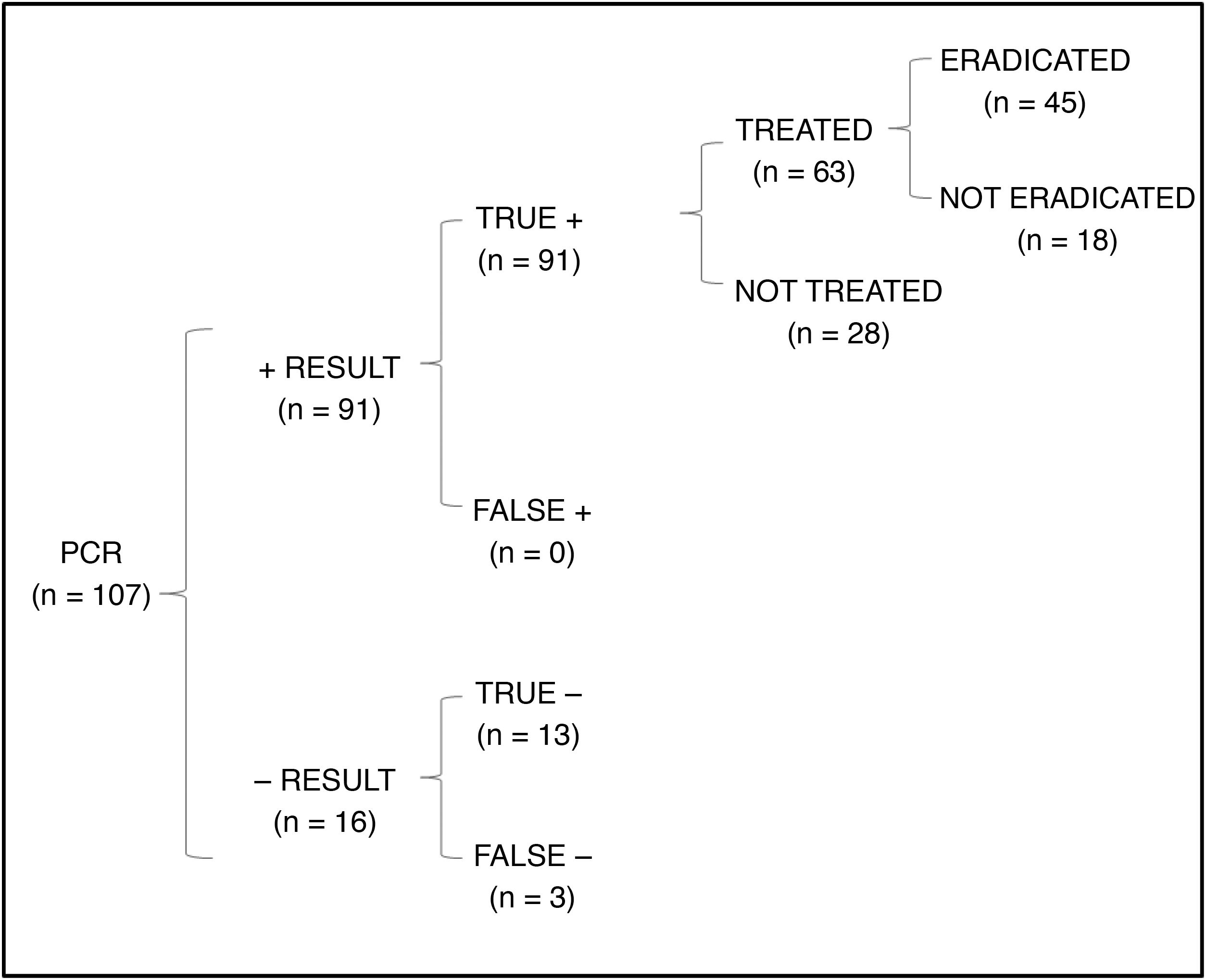
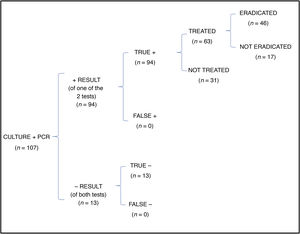
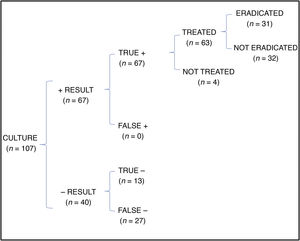
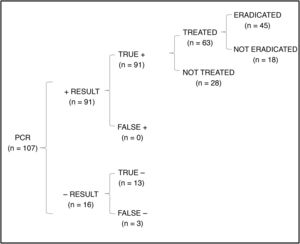
In the cost-effectiveness analysis, of the total of 107 collected samples, 13 turned out negative with both techniques, 64 were positive in both culture and PCR tests, 27 were negative in the culture and positive in the PCR test and 3 were positive in the culture and negative in the PCR test. Fig. 1 shows the results of analysing the number of eradicated cases in the total sample, while the outcomes of the 2 hypothetical scenarios are presented in Fig. 2 (only culture) and Fig. 3 (only PCR). Table 2 presents the total costs of each option. The most cost-effective strategy was the use of PCR testing alone, with a CER of 71.91 compared to 92.16 for culture and 96.35 for culture+PCR.
In the paediatric age group, the decision to treat H. pylori should be individualised and considered in patients that are symptomatic or have complications.7,20 Since 2011, the ESPGHAN and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) consider antibiotic regimens lasting 7–14 days guided by antibiotic susceptibility testing or untargeted strategies like sequential therapy valid.20 The guidelines published in 2017 only consider regimens lasting 14 days and guided by antimicrobial susceptibility testing acceptable.7
In cases in which eradication is indicated, the infection should be confirmed by at least 2 direct diagnostic tests performed on specimens of gastric mucosa (direct visualization in histological exam, culture or PCR),15–17 avoiding the test and treat approach.14
An analysis of data from the EuroPedHP Registry4 found a percentage of eradication for short regimens lasting 7–10 days of 75.6% compared to 85% with long regimens lasting 14 days. In our study, while the efficacy of regimens that adhered to the 2017 recommendations of the ESPGHAN7 was greater compared to the success observed with the 2011 recommendations,20 the frequency of successful eradication was not as high. These differences could be explained by the smaller sample size of our study in addition to the large percentage of patients lost to follow-up. The target success rate of 90% has not been achieved at any time.7
Antibiotic resistance is the leading cause of treatment failure in the management of infection by H. pylori.11 In recent decades there has been evidence of an increasing trend in the prevalence of resistance to the main antibiotics used in its treatment.7 Among the causes that have been proposed are an increase in the consumption of macrolides in Spain, other countries in Southern Europe and the Western Pacific Region, the regions where resistance to these drugs is most prevalent.12,13 This, combined with the use of test and treat approaches14 without previous direct confirmation or microbiological testing, could account for the observed increases in antibiotic resistance.
In our study, we found a decreased prevalence of resistance to clarithromycin in the first period (2013−2016) compared to the previous literature.9,10 However, between 2017 and 2019, the prevalence rose to 30.8% based on culture results and up to 53.2% after the introduction of PCR testing. While culture detected 60% of resistance cases in the second period, the introduction of PCR testing allowed detection of 98%. The higher sensitivity of molecular techniques17,19 could explain the increase in the observed prevalence, although we have no way of knowing if underdetection in the previous period played a role in it. None of the included patients had ever received eradication therapy, so all the cases of drug resistance were considered primary, and we could not draw conclusions regarding secondary resistance. When it came to antibiotics other than clarithromycin, the proportions of resistance were similar in both periods, although lower compared to the prevalence described in previous European studies in the case of metronidazole, which ranged from 20% to 40%.10,11 The prevalence of ampicillin resistance in our sample was all but negligible.
In the cost-effectiveness analysis of the different diagnostic strategies, using culture alone was the least costly option, but it was associated with a lower frequency of successful eradication (49.2%). The use of PCR testing alone was the most cost-effective option, with a frequency of successful eradication of 71.4%, very close to the frequency achieved with the use of both culture and PCR testing, but with a lower cost. However, culture does offer added value as it can be used to detect resistance to other antibiotics.15,16
Some of the limitations of our study are its retrospective design, the losses to follow-up and the lack of monitoring of adherence to treatment and the degree of adherence in cases in which eradication failed. Since PCR testing was not used in the first period, we cannot determine whether the observed increase in drug resistance was due to underdiagnosis in the earlier period or an actual increase in prevalence in the later period. Prospective studies are required to elucidate this issue.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Gallardo Padilla M, León Falconi JL, Sánchez-Nebreda Arias R, Gómez Santos C, Muñoz Egea MdC, Orden Izquierdo El. Impacto del uso de las técnicas moleculares (PCR) en la detección y el éxito erradicador frente a Helicobacter pylori. An Pediatr (Barc). 2022;96:190–195.