To assess the effect of a protocolised intervention for low systemic blood flow (SBF) in the occurrence of severe intraventricular haemorrhage (IVH) or death in pre-term infants.
MethodsA study with a quasi-experimental design with retrospective controls was conducted on pre-term infants of less than 30weeks of gestational age, born between January 2016 and July 2017, who were consecutively included in the intervention period. The control cohort included pre-term infants (born between January 2013 and December 2015) matched by gestational age, birth weight, and gender (two controls for each case). The cases of low SBF diagnosed according to functional echocardiography during the study period received dobutamine (5–10μg/kg/min) for 48h.
ResultsThe study included 29 cases (intervention period) and 54 controls (pre-intervention period). Ten out of 29 (34.5%) infants received dobutamine for low SBF during the intervention period, with 3/29 (10.3%) cases of severe IVH and/or death compared to 17/54 (31.5%) in the control cohort (P=.032). There was an independent association between the intervention and a decreased occurrence of severe IVH/death after adjusting for confounding factors both in the logistic regression model [OR 0.11 (95%CI: 0.01–0.65), P=.015], as well as in the sensitivity analysis using inverse probability of treatment weighting [OR 0.23 (95%CI: 0.09–0.56); P=.001].
ConclusionsIn this study with retrospective controls, a protocolised screening, and treatment for low SBF was associated with a decreased occurrence of severe IVH or death in preterm infants. Large, adequately powered trials, are needed in order to determine whether postnatal interventions directed at low SBF can improve neurological outcomes.
Evaluar el efecto de un protocolo de cribado y tratamiento del bajo flujo sistémico (BFS) durante el periodo transicional en la aparición de hemorragia intraventricular (HIV) grave y/o muerte en prematuros.
MétodosEstudio cuasi-experimental con controles retrospectivos. En la fase de intervención se incluyeron los prematuros de menos de 30semanas de edad gestacional (enero 2016-julio 2017). Los controles (enero de 2013-diciembre de 2015) fueron pareados por edad gestacional, peso al nacimiento y sexo con una relación 1:2. Los casos diagnosticados de BFS por ecocardiografía funcional durante el protocolo recibieron tratamiento con dobutamina (DB) entre 5-10μg/kg/min durante 48h.
ResultadosSe incluyeron 29 casos en la fase de intervención (aplicación del protocolo) y 54 controles (fase preintervención). Diez de 29 (34,5%) casos durante el protocolo recibieron DB por BFS con 3/29 (10,3%) casos de HIV grave y/o muerte comparado con 17/54 (31,5%) en la fase pre-protocolo (p=0,032). El protocolo se asoció de forma independiente a una reducción en la HIV grave y/o muerte tanto en la regresión logística (OR: 0,11 [IC95%: 0,01-0,65], p=0,015] como en el análisis de ponderación por la probabilidad inversa de tratamiento (OR: 0,23 [IC95%: 0,09-0,56]; p=0,001].
ConclusionesEn un estudio con controles retrospectivos, la aplicación de un protocolo de cribado y tratamiento del BFS en prematuros se asoció a una reducción en la HIV grave y/o muerte. Son necesarios ensayos clínicos de suficiente potencia para determinar si las intervenciones posnatales sobre el BFS pueden mejorar el pronóstico neurológico.
During the period of perinatal transition, preterm newborns may experience a transient circulatory deterioration characterised by low systemic blood flow (LSBF).1,2 The immaturity of the myocardium of preterm newborns may contribute to the development of low systemic blood flow, along with other factors such as inflammation (chorioamnionitis, sepsis), hypoxemia or changes in preload (mechanical ventilation, patent ductus arteriosus [PDA], etc.). Techniques like functional echocardiography or measurement of regional oxygen saturation allow the detection of abnormalities in systemic and cerebral blood flow that would otherwise go unnoticed.3–5 The clinical signs used routinely for monitoring (heart rate, blood pressure, etc.) are not sensitive enough to detect changes in systemic blood flow.2,5–8 Furthermore, other indirect markers of perfusion, such as serum lactate levels or urinary output, are of little usefulness in extremely preterm newborns during the transitional period.9 Low systemic blood flow tends to be transient and resolve spontaneously in the first 24–48h of life.10 However, there is evidence that this period of relative hypoperfusion may be involved in the aetiology of brain injury, with a demonstrated association between a superior vena cava flow (SVCf) of less than 41mL/kg/min and intraventricular haemorrhage (IVH), ischaemia-induced white matter injury, long-term neurodevelopmental impairment and death.1,3,11–15
There are interventions, such as delayed cord clamping or umbilical cord milking, that may reduce the risk of IVH.1,16–19 Some authors have proposed the use of inotropic drugs for prevention and treatment of LSBF during the transitional period, although there is no clear evidence of their impact on mortality or long-term developmental outcomes. Unfortunately, none of the studies conducted to date was sufficient powered, so no recommendations can be made on this subject.20–22
There is published evidence on the use of functional echocardiography in some neonatal units in Spain, but we do not know whether routine implementation of this method in everyday practice would have an impact on clinical outcomes.23
The aim of this study was to assess the impact of implementing a protocol for screening of LSBF and selective treatment with dobutamine (DB) during the transitional period on the incidence of severe IVH and/or mortality in low-birth-weight preterm newborns.
MethodsDesign and sampleWe conducted a quasi-experimental, pre-post intervention study to analyse the impact of the introduction of a LSBF screening and treatment protocol. We included consecutively all newborns delivered preterm before 30 weeks’ gestational age (GA) after the introduction of the protocol (from January 1, 2016 to July 1, 2017). For the control group, we selected a retrospective cohort of newborns delivered preterm before the protocol was implemented (January 1, 2013 to December 31, 2015) matched for GA (±3.5days), birth weight (±100g) and sex at a 1:2 ratio. We excluded newborns with major malformations, congenital heart disease or incomplete clinical data. We excluded patients with IVH detected in the first 6h post birth prospectively.
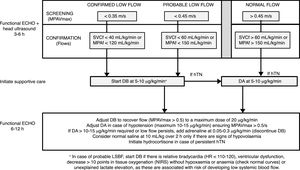
Intervention phase protocolAt 3–6h post birth, newborns were assessed by functional echocardiography to measure systemic blood flow at the time the first head ultrasound (see below) was performed for early detection of IVH. Two neonatologists especially trained in functional echocardiography and one paediatric cardiologist performed the echocardiographic examinations. In all cases, the assessment of LSBF included measurement by pulsed-wave Doppler of the peak velocity in the main pulmonary artery (MPAVmax), in addition to blood flow in the main pulmonary artery (MPAf) and in the superior vena cava (SVCf). Confirmed LSBF was defined as values of MPAVmax of 0.35 m/s or less, MPAf of 120mL/kg/min or less, or SVCf of 40mL/kg/min or less. Probable LSBF was defined as values of MPAVmax between 0.35 and 0.45m/s, MPAf between 121 and 150mL/kg/min and SVCf between 41 and 60mL/kg/min. As specified in the protocol, newborns received DB starting at a dose of 5–10μg/kg/min in case of confirmed LSBF or of probable LSBF with additional risk factors (Fig. 1). The treatment was maintained until 48h post birth. The dose of DB was adjusted based on the findings of a second functional echocardiogram performed to assess the effect of the drug 6–12h after the first dose.
Protocol for the screening and treatment of low systemic blood flow and haemodynamic support in the transitional period in preterm newborns delivered before 30 weeks’ gestation. DA, dopamine; DB, dobutamine; hTN, arterial hypotension; MPAVmax, main pulmonary artery peak velocity; MPAf, main pulmonary artery blood flow; SVCf, superior vena cava blood flow.
The institutional protocol for the management of arterial hypotension (hTN) was the same in the 2 periods under study. The criterion for diagnosis of hypertension was a mean arterial pressure (MAP) below the 5th percentile for GA and post birth age in hours.24 The first-line drug for treatment of hTN was dopamine (DA). If the patient required DA at doses of more than 10–15μg/kg/min, adrenaline was added at a dose of 0.05–0.3μg/kg/min. Boluses of normal saline at a dose of 10 cc/kg were given only to patients with persistent hTN if there was evidence of hypovolaemia and based on the judgment of the physician in charge. Hydrocortisone (1mg/kg/6–8h) was used to treat hTN refractory to previous treatments.25–28 In our hospital, we did not practice delayed cord clamping or umbilical cord milking in preterm births during the period under study. During the intervention period, there were some changes in clinical practice, such as the introduction of the administration of probiotics to prevent enterocolitis, an update to the protocol for administration of surfactant based on the European consensus guidelines of 2016,29 an update to the protocol for administration of hydrocortisone for prevention of bronchopulmonary dysplasia,30 the introduction of the routine use of magnesium sulphate and the increase in the proportion of women at risk of preterm birth that received full courses of corticosteroids to accelerate foetal lung maturation.
Outcome variablesWe collected data on clinical variables and the associated morbidity in all patients through discharge from the unit. All patients underwent a head ultrasound examination at 3–6h, at 3, 7 and 28 days post birth and at discharge, in adherence with the protocol of the unit. The primary outcome of the study was a composite of the presence of severe IVH (grades 3–4 in the Papile classification) or death, which we combined on account of them being competitive events.
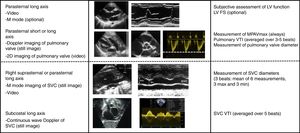
Methods used for measurement of systemic blood flowWe adhered to published recommendations in the measurement of systemic blood flow.31 The investigators measured the MPAVmax at the level of the pulmonary valve from the parasternal short- or long-axis view with pulsed-wave Doppler. We calculated the peak velocity (in m/s) as the mean peak velocity over 3–5 heart beats (Fig. 2). To measure pulmonary flow, we calculated the velocity-time integral (VTI) of the Doppler signal averaged over 3–5 beats. We calculated pulmonary blood flow based on the measurement of the diameter of the pulmonary annulus at the end of systole with the formula (D/2)2×VTIm×π×HR/weight, where D stands for the diameter of the pulmonary valve, VTIm for the VTI averaged over 3–5 heart beats, and HR for the heart rate. We expressed the result in mL/kg/min. The measurement of the SVCf was obtained with the same method. The diameter of the vena cava was measured using the M mode technique from a modified suprasternal or parasternal view. Due to the significant respiratory variation in the diameter of the superior vena cava (SVC), we used the mean of 3 maximums and 3 minimums diameters to calculate the flow. The SVCf VTI was measured from a subcostal view and averaged over 5 heart beats (Fig. 2).
Functional echocardiography protocol during the period under study. We specify the echocardiographic windows included in the examination and the parameters that had to be recorded for each. FS, fractional shortening; LV, left ventricle; MPAVmax, main pulmonary artery peak velocity; SVC, superior vena cava; VTI, velocity-time integral.
We summarised quantitative data as median and interquartile range or mean and standard deviation, and categorical data as absolute frequencies and percentages. We made comparisons using nonparametric tests (Mann-Whitney U, McNemar, Wilcoxon), the χ2 test, the Fisher exact test or the Student t test as appropriate. We performed a multivariate logistic regression analysis to assess the association between the protocol and the incidence of severe IVH (grade 3 or 4) and/or death (Table 1). Due to the risk of overadjustment in logistic regression, we performed a sensitivity analysis using inverse probability treatment weighting based on propensity scores. To estimate propensity scores, we included covariates that could have an influence the development of IVH (GA, weight, sex, CRIB score at 12h, chorioamnionitis, prenatal steroid exposure, type of delivery, Apgar at 5min, low blood pressure, vasoactive-inotropic score, transfusion, days of mechanical ventilation, haemodynamically significant PDA and sepsis).32 We assessed covariate balance by calculating standardised differences, and defined adequate balance as a standardised difference of less than 10%.33 We assessed the effect of the intervention by means of generalised estimating equations regression.
Patient characteristics and outcomes.
| Pre-protocol (n=54) | Protocol (n=29) | P | |
|---|---|---|---|
| Clinical characteristics | |||
| Sex (male) | 35/54 (64.8%) | 19/29 (65.5%) | .949 |
| Gestational age (weeks) | 27.6 (26.2–29.2) | 27.4 (26.3–29.1) | .583 |
| Birth weight (g) | 990 (800–1185) | 985 (745–1175) | .886 |
| Twin delivery | 12/54 (22.2%) | 9/29 (31%) | .379 |
| Apgar 5 min | 8 (7–9) | 8 (6–9) | .465 |
| Magnesium sulphate | 5/52 (9.6%) | 15/29 (51.7%) | .000 |
| Preeclampsia | 10/54 (18.5%) | 2/29 (6.8%) | .151 |
| In vitro fertilisation | 4/54 (7.4%) | 7/29 (24.1%) | .032 |
| Maternal chorioamnionitis | 9/54 (16.6%) | 4/29 (13.7%) | .731 |
| Prenatal steroids | |||
| No | 6/54 (11.1%) | 2/29 (6.8%) | |
| Incomplete | 15/54 (27.7%) | 4/29 (13.7%) | .236 |
| Complete | 33/54 (61%) | 23/29 (79.3%) | |
| Caesarean delivery | 38/54 (70.3%) | 17/29 (58.6%) | .280 |
| RDS | 38/54 (70.3%) | 22/29 (75.8%) | .584 |
| Number of surfactant doses | 2 (1–3) | 1 (1–2) | .078 |
| Days of intubation | 2.2 (0–9) | 3 (0–8) | .950 |
| CRIB 12 h | 2 (1–4.2) | 4 (1–7) | .232 |
| Transfusion | 39/54 (72.2%) | 16/29 (55%) | .117 |
| Number of transfusions | 1 (1–2) | 2 (1.2–2.7) | .021 |
| Early-onset sepsis | 4/54 (7.4%) | 2/29 (6.8%) | .932 |
| Early hypotension (<5 days) | 10/54 (18.5%) | 7/29 (24%) | .574 |
| Vasoactive-inotropic score | 190 (65–296) | 180 (100–220) | .962 |
| Hydrocortisone | 8/54 (14.8%) | 3/29 (10.3%) | .546 |
| Late-onset sepsisa | 16/54 (29.6%) | 11/28 (39.2%) | .478 |
| Hs PDA (treated) | 23/54 (42.5%) | 10/29 (34.4%) | .472 |
| Enterocolitis | 7/47 (14.8%) | 1/26 (3.8%) | .245 |
| Retinopathy | 5/45 (11%) | 1/26 (3.8%) | .404 |
| BPD (moderate-severe) | 12/45 (26.6%) | 8/26 (30.7%) | .711 |
| Outcomes | |||
| IVH of any grade | 21/53 (39.6%) | 8/29 (27.5%) | .157 |
| IVH grades 3–4 | 11/53 (20.7%) | 3/29 (10.3) | .100 |
| Death | 10/54 (18.5%) | 3/29 (10.3%) | .360 |
| IVH 3–4 and/or death | 17/54 (31.5%) | 3/29 (10.3%) | .032 |
We calculated the vasoactive-inotropic score as: 10×dopamine+10×dobutamine+100×adrenaline+100 noradrenaline, using the maximum values in the first 5 days of life in patients that received vasoactive drugs.
Refers to clinically suspected sepsis (not necessarily confirmed by microbial isolation) in which empirical antibiotherapy was initiated.
BPD, bronchopulmonary dysplasia, CRIB, Clinical Risk Index for Babies score; Hs PDA, haemodynamically significant patent ductus arteriosus; IVH, intraventricular haemorrhage; RDS, respiratory distress syndrome.
We assessed the linear correlation between the MPAVmax and the SVCf with the Pearson correlation coefficient. Before conducting the study, we assessed the interrater agreement between the 3 investigators that were to perform the echocardiographic examinations in the study, comparing their measurements of the MPAVmax in 25 examinations performed in 15 newborns (GA, 27–32 weeks) that were not included in the protocol period.
EthicsThe study protocol was approved by the competent Board of Ethics and Clinical Research. We obtained informed consent for the patients included in the intervention group (implementation of the protocol). The control group was selected retrospectively, and the Board waived the need for informed consent in these patients.
ResultsIn the first 18 months following the introduction of the protocol, it was applied to 30 patients, whom we matched to 60 controls. We excluded 1 case and 6 controls for different reasons: prospective exclusion of 3 patients due to diagnosis of IVH in the first 6h (1 case and 2 controls), and exclusion of 4 eligible patients due to missing clinical data. Thus, the final analysis included 29 cases and 54 controls.
In the total sample, the GA at birth was 27.5 weeks (26.2–29.1) and the weight 990g (800–1180). Table 1 compares the characteristics of the patients in the two groups. We found no significant differences between groups in the variables under study except in the prenatal administration of magnesium sulphate, the proportion of pregnancies resulting from in vitro fertilisation and the number of transfusions received.
In the period that the protocol was implemented, 3/29 patients (10.2%) developed severe IVH or died, compared to 17/54 (31.5%) in the pre-intervention period (P=.032). There was a statistical trend to decreased incidence of severe IVH in the protocol period (3/29 [10.3%] vs 11/53 [20.7%]; P=.100), although we did not find significant differences in the incidence of overall IVH of any grade. A total of 13 patients died (3 in the protocol period and 10 in the pre-intervention period). In 3 patients (2 cases and 1 control), the cause of death was withdrawal of treatment due to severe IVH.
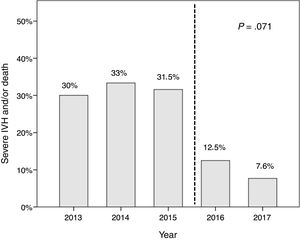
We found a decreasing linear trend in severe IVH and/or death that coincided with the implementation of the protocol (P=.071) (Fig. 3). We did not find this trend in the incidence of retinopathy, haemodynamically significant PDA, moderate-to-severe bronchopulmonary dysplasia or nosocomial sepsis, although we did find a decreasing trend in the incidence of enterocolitis (P=.093).
In the multivariate logistic regression analysis, the implementation of the protocol was associated with a significant reduction in the incidence of severe IVH and/or death (OR, 0.11 [95% CI, 0.01–0.65], P=.015) after adjusting for potential confounders (Table 2). When we performed sensitivity analysis using inverse probability treatment weighting, we obtained a sample with an adequate balance on all the covariates included in the model (range of standardised differences, −8.6% to +8%). In the analysis of the weighted sample, the LSBF protocol was associated with a reduction in the incidence of severe IVH and/or death (OR, 0.23 [95% CI, 0.09–0.56]; P=.001).
Multivariate logistic regression model (dependent variable: severe IVH and/or death).
| Predictors | Univariate analysis | Multivariate analysis (final model) | |||
|---|---|---|---|---|---|
| OR (95% CI) | P | Predictors | OR (95% CI) | P | |
| LSBF protocol | 0.25 (0.06–0.94) | .041 | LSBF protocol | 0.11 (0.01–0.65) | .015 |
| Birth weight (z) | 0.39 (0.19–0.76 | .006 | Birth weight (z) | 0.19 (0.04–0.70) | .022 |
| Birth weight (kg) | 0.034 (0.00–0.38) | Birth weight (kg) | 0.03 (0.0–0.43) | ||
| GA (z) | 0.48 (0.27–0.86) | Chorioamnionitis | |||
| GA (weeks) | 0.65 (0.46–0.91) | .014 | 9.48 (1.39–64.5) | .021 | |
| Chorioamnionitis | 3.42 (0.99–11.8) | .051 | RDS | 16.5 (1.42–188) | .024 |
| CRIB at 12 h (z) | 1.75 (1.06–2.87) | .026 | Arterial hypotension | 6 (1.06–33.7) | .042 |
| RDS | 10.19 (1.27–81.2) | .028 | |||
| Number of surfactant doses | 2.54 (1.43–4.51) | .001 | |||
| Arterial hypotension | 4.36 (1.37–13.81) | .012 | |||
| VIS (z) | 3.1 (1.64–5.8) | <.001 | |||
| Transfusion | 3.72 (0.98–14.05) | .052 | |||
Variables included in the univariate analysis: study group (LSBF protocol), GA, birth weight, sex, in vitro fertilisation, twin delivery, prenatal corticosteroids, type of delivery, maternal chorioamnionitis, prenatal magnesium sulphate, maternal preeclampsia, Apgar 5min, CRIB 12h, days of mechanical ventilation, RDS, doses of surfactant, arterial hypotension (<7 days post birth), vasoactive-inotropic score (VIS) (<7 days), haemodynamically significant PDA, transfusion, number of transfusions, early-onset sepsis. Variables corresponding to P-values of less than 0.1 in the univariate analysis were considered for inclusion in the multivariate regression model. Other variables potentially associated with severe IVH (haemodynamically significant PDA, prenatal magnesium sulphate, prenatal corticosteroids, type of delivery, in vitro fertilisation) were included in the model one by one. Due to their strong correlation, each variable in the pairs birth weight-GA, hypotension-VIS and RDS-doses of surfactant was introduced in the models separately from the other to avoid colinearity. Continuous variables were standardised as z-scores (z) to facilitate the interpretation of the odds ratios. The final model only included those variables that continued to have a statistically significant association.
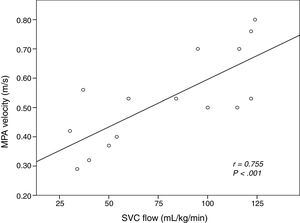
The intraclass correlation coefficient comparing the investigators that measured MPAVmax by means of echocardiography was 0.992 (95% CI, 0.988–0.996; P<.001). During the intervention period, systemic blood flow was assessed by measurement of the MPAVmax in all patients, supplemented by measurement of the SVCf in 15 patients and the MPAf in 9 patients. Fig. 4 shows the linear correlation between the MPAVmax and the SVCf.
In the protocol period, 10/29 patients (34.5%) received a diagnosis of LSBF, which was made at a median of 4h post birth (IQR, 2–10), and were treated with DB starting with a dose of 6μg/kg/min (IQR, 5–10) (Table 3). We found no difference between these patients compared to patients that did not receive DB in GA, birth weight, Apgar score at 5min, incidence of respiratory distress syndrome (RSD) or duration of mechanical ventilation in days; however, they did have a significantly higher CRIB score at 12h post birth (7 [4–9.2] vs 1 [1–5]; P=.03). After initiation of DB, there was a significant increase in systemic blood flow (MPAVmax pre-DB, 0.40 m/s [0.32–0.42] vs post-DB, 0.60 m/s [0.53–0.60]; P=.018). None of the patients treated with DB developed severe IVH or died. Conversely, 3 patients in the group that did not receive DB developed severe IVH. The values of MPAVmax in these patients ranged between 0.47 and 0.53 m/s, compared to a median of 0.60 m/s (IQR, 0.52–0.80) in patients that did not develop IVH. Out of the patients that did not receive DB, functional echocardiography was performed to assess changes in systemic blood flow in only 10 (data not available for the 3 patients with severe IVH).
Values of the parameters measured by functional echocardiography in patients in the protocol group.
| 1st functional echocardiogram | Dobutamine (n=10) | No dobutamine (n=19) | P |
|---|---|---|---|
| MPAVmax (m/s) | 0.39 (0.07) 0.40 (0.32–0.43) | 0.59 (0.11) 0.53 (0.51–0.70) | <.001 |
| SVC flow (mL/kg/min) | 40.8 (9.2) 38.5 (33–51) | 104.2 (21.6) 115 (89–122) | <.001 |
| Pulmonary flow (mL/kg/min) | 209 (162) 148 (124–324) | 298 (36) 309 (260–326) | .190 |
| 2nd functional echocardiogram | Dobutamine (n=10) | No dobutamine (n=10) | P |
|---|---|---|---|
| MPAVmax (m/s) | 0.57 (0.04) 0.60 (0.53–0.60)* | 0.65 (0.01) 0.69 (0.61–0.72)** | .267 |
| SVC flow (mL/kg/min) | 81 (22) 81 (65–100) | 107 (41) 107 (78–119) | .429 |
| Pulmonary flow (mL/kg/min) | 297 (95) 260 (217–395) | 345 (156) 345 (235–401) | .857 |
The echocardiographic parameters follow normal distributions and are given as mean (SD) and median (IQR). Variables were compared between groups using the Mann–Whitney U test.
MPAVmax, main pulmonary artery peak velocity; SVC, superior vena cava.
In this study, we assessed the impact of the implementation of a protocol for screening for LSBF by means of functional echocardiography and its selective treatment with DB in the first 18 months from its introduction. We found a decrease in the incidence of severe IVH and/or death that was not associated with any adverse events.
The gold standard for diagnosis of LSBF is measurement of blood flow in the superior vena cava by means of echocardiography.4,34,35 However, this is a technically complex measurement, and thus may be difficult to implement in everyday clinical practice. The main source of error is the measurement of the diameter of the vena cava, as this value is squared to calculate the flow, and some authors have questioned the validity of this method for the purpose of clinical decision-making.31,35–38 Measurement of the MPAVmax has been proposed as an alternative for estimating systemic blood flow. The MPAVmax is proportional to cardiac output and is strongly associated with systemic blood flow in the first days of life, when the volume diverted through the foramen ovale is small. It is much easier to obtain than the SVCf and does not require any calculations or measurement of dimensions that increase the risk of error. A peak velocity of less than 0.35 m/s is indicative of LSBF in most patients, while about half of patients with a peak velocity of less than 0.45 m/s may have LSBF.39
Despite the mounting evidence on the association of LSBF with brain injury and mortality, its prevention and treatment through the use of inotropic agents remains controversial. In 2007, Osborn et al.13 demonstrated that treatment with DB is more efficacious in increasing systemic blood flow and reducing the incidence of IVH compared to dopamine, while a different clinical trial did not find significant increases in systemic blood flow with the use of milrinone.21 However, there is no evidence that the use of inotropes is associated with an improvement of long-term neurologic outcomes. Unfortunately, none of the studies conducted to date had sufficient power to detect differences in morbidity and mortality.20 In 2015, Bravo et al.40 conducted a pilot randomised-controlled trial where they assigned preterm newborns with LSBF (SVCf<41mL/kg/min) to either DB (n=16) or placebo (n=12). The study also included a control group of 98 preterm newborns with normal SVCf values. They found that LSBF was associated with higher mortality and an increased incidence of severe ischaemic events. The incidence of IVH was 12% in the DB group compared to 33% in the placebo group. Although the differences were not statistically significant due to the low statistical power of the study, its results were very similar to our own findings. We believe that a larger study could find significant differences in the incidence of this important clinical event.
Our results were promising but must be interpreted taking into account the limitations of our study. First of all, it was a single-centre study with a retrospective control group. We cannot be completely sure that the decrease in the incidence of severe IVH resulted solely from the implementation of the LSBF management protocol. Factors like additional improvements in clinical practice during the period under study or the possibility that more attention or care was given to the patients in the intervention group (Hawthorne event) may have influenced the outcomes. We attempted to minimise these effects intrinsic to the study design by selecting matched controls and analysing potential confounders. We did not find statistically significant differences in most of the baseline characteristics or in morbidity between the groups, which suggests that the 2 cohorts were comparable. The incidence of other morbidity, which could indicate an overall improvement in care, did not decrease during the period under study, whereas we did observe a decreasing trend in the incidence of IVH that coincided with the implementation of the protocol. Lastly, the multivariate regression analysis and the model using inverse probability treatment weighting found an independent association between the protocol and the decrease in severe IVH and/or death after adjusting for potential confounding factors.
The diagnosis of LSBF and the prescription of DB were mainly based on the measured value of the MPAVmax. There are no validation studies of MPAVmax against SVCf, which is considered the gold standard for diagnosis. Nevertheless, we found a strong linear correlation between the MPAVmax and the SVCf, which needs confirmation by studies specifically focused on this aspect. In addition we found an excellent interrater agreement in the measurement of the MPAVmax. Our protocol did not include monitoring of changes in blood flow in patients that did not receive DB. In fact, the three IVH events occurred in patients in this group. These newborns had initial values of MPAVmax ranging between 0.47 and 0.53m/s, which were barely above the threshold we had established for indication of DB. It is possible that these patients had LSBF that went undetected. For this reason, we consider that monitoring changes in blood flow with functional echocardiography is crucial in these patients, especially when the initial values are near the threshold for treatment.
Performance of prospective multicentre studies, preferably clinical trials, with sufficient statistical power is necessary to establish whether interventions in the management of LSBF such as administration of inotropes have a favourable impact on the prevention of IVH and long-term neurologic outcomes.
ConclusionIn this study with retrospective controls, the implementation of a protocol for the detection and selective treatment of LSBF during the transitional period was associated with a reduction in the incidence of severe IVH/death in preterm newborns delivered before 30 weeks’ gestation. Due to the study design, we were unable to rule out the potential contribution of other factors to the observed improvement.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Oulego Erroz I, Alonso Quintela P, Jiménez Gonzalez A, Terroba Seara S, Rodríguez Blanco S, Rosón Varas M, et al. Impacto del cribado y tratamiento del bajo flujo sistémico en la prevención de hemorragia intraventricular grave y/o muerte en el prematuro. An Pediatr (Barc). 2018;89:369–377.
Previous presentation: This study was presented at the XXVI Spanish Congress of Neonatology and Perinatal Medicine; September 27–29, 2017, Zaragoza, Spain.