Vitamin D is an essential prohormone in calcium and phosphorus homeostasis. Recent studies show a high frequency of insufficiency/deficiency of vitamin D in the general population worldwide. Our objective was to estimate the prevalence of circulating vitamin D [25(OH)D3] deficiency and insufficiency in children and examine the associated factors.
Material and methodsA total of 283 children, participants in the cohort INMA-Asturias, were studied. The 25(OH)D3 concentrations were quantified by high performance liquid chromatography. The prevalence of deficiency [25(OH)D3<20ng/mL] and insufficiency [20–29.9ng/mL] of vitamin D was estimated. Distribution of 25(OH)D3 for month of extraction of specimen, ingestion, and other factors were analysed.
ResultsThe mean 25(OH)D3 was 20.1ng/mL (range 2.7–49.8), with 8.8% ≥30ng/mL, 38.5% from 20 to 20.9ng/mL, and 52.7%<20ng/mL. Seasonal variation was found, with lower values in winter. There was no relationship between plasma levels and intake of vitamin D (median 2.7μg/day, range 0.81–12.62), time outdoors (mean 3h, range: 0:21–6:55), or BMI or gender, but there was one found with the mother's levels during gestation.
ConclusionsThere is a high prevalence of vitamin D deficiency/insufficiency in children at 4 years. Solar exposure might not be enough in our region. Healthy children should be encouraged to follow adequate outdoor activities with associated sun exposure. Due to the deficit of intake in childhood, recommendations are needed about a varied diet with vitamin D-containing foods in this age group, especially during the winter, and assessing the need of vitamin D supplementation in children at risk.
La vitamina D es una prohormona esencial en la homeostasis del calcio y el fósforo. Estudios recientes muestran una elevada frecuencia de insuficiencia/deficiencia de vitamina D en población general a nivel mundial. Nuestro objetivo ha sido estimar la prevalencia de deficiencia e insuficiencia de vitamina D sérica [25(OH)D3] y examinar sus factores asociados en la infancia.
Material y métodosSe ha estudiado a 283 niños participantes en la cohorte INMA-Asturias. Se determinó la 25(OH)D3 mediante cromatografía líquida de alta resolución. Se han estimado las prevalencias de deficiencia ([25(OH)D3<20ng/ml) e insuficiencia (20-29,9ng/ml) de vitamina D y se ha analizado la distribución de 25(OH)D3 por mes de extracción, ingesta y otros factores.
ResultadosLa 25(OH)D3 media fue 20,1ng/ml (rango 2,7-49,8). El 8,8% tenía 25(OH)D3≥30ng/ml, el 38,5% entre 20-20,9ng/ml y el 52,7%<20ng/ml. Se halló variación estacional con menores valores en invierno. No se encontró relación entre los niveles plasmáticos y la ingesta de vitamina D (mediana 2,7μg/día, rango 0,81-12,62), el tiempo al aire libre (mediana 3h, rango: 0:21-6:55), el índice de masa corporal, ni el sexo, pero sí con los niveles de sus madres durante la gestación.
ConclusionesExiste una elevada prevalencia de deficiencia/insuficiencia de vitamina D a los 4 años. La exposición solar podría no ser suficiente en nuestra región. Se deberían promover actividades al aire libre con una adecuada exposición a la luz solar. Dado el déficit de ingesta en la infancia, es necesario hacer recomendaciones de una alimentación variada rica en vitamina D en este periodo especialmente durante el invierno, valorando la necesidad de suplementar con vitamina D en los niños de riesgo.
Vitamin D, the sun vitamin, is known for its importance in bone health. But this is just the tip of the iceberg, as it is also associated with other health benefits, including decreasing the risk of chronic pathologies such as autoimmune, neurologic or cardiovascular disease or cancer, among others.1–7
Low levels of vitamin D during pregnancy and childhood are associated with an increased risk of complications in pregnancy and foetal growth and development (preeclampsia, gestational diabetes, small for gestational age, impaired foetal neurologic development, etc.),8,9 as well as severe asthma, decreased response to corticosteroids, type 1 diabetes, rheumatoid arthritis, cancer and cardiovascular disease,3,5,10,11 not to mention, of course, the resurgence of rickets.12
Recent population-based studies have found a high prevalence of low serum levels of vitamin D [25(OH)D3] worldwide, including in children.13,14
There is no universal consensus on the optimal 25(OH)D3 serum levels needed to achieve an adequate bone mineralisation, and there is even less agreement on the levels required for the other functions of this prohormone.15–17 Thus, while the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) and the Institute of Medicine (IOM) of the United States define sufficiency as levels of 20ng/dL and greater, the Endocrine Society (Holick et al., 2011) defines deficiency as less than 20ng/mL (50nmol/L), insufficiency as 20–29ng/mL (50–74nmol/L) and sufficiency as 30ng/mL or more (75nmol/L). There are also studies that claim that disturbances in calcium absorption and low bone mineral density are associated with levels below 32ng/mL.18 In this study, we applied the criteria of the Endocrine Society.
Our aim was to estimate the prevalence of serum vitamin D deficiency and insufficiency and examine the factors associated with vitamin D deficiency and insufficiency in children aged 4 years in the Asturias cohort of the INMA Project (Infancia y Medio Ambiente [Childhood and Environment]).
Materials and methodsSample under studyWe analysed data for 283 children in the Asturias cohort of the Universidad de Oviedo that is part of the INMA Project.19 The INMA Project is a prospective population-based cohort study whose primary objective is to study the effects of diet and exposure to pollutants during pregnancy and early childhood in health and development from foetal life through adolescence and adulthood in Spain (www.proyectoinma.org).
The study was approved by the regional research ethics committee of the Principality of Asturias. All eligible pregnant women were given verbal and written information about the project, and participants were included after signing an informed consent form.
Measurement of 25(OH)D3The samples were processed immediately and stored at −70 to −80°C until they were analysed. Serum levels of 25(OH)D3 were determined by high performance liquid chromatography with BioRAD, adhering to clinical protocols and Standard Institute Laboratory guidelines. The limit of detection was 5ng/mL and the inter-assay coefficient of variability was 4.5%. The assay was validated by means of a German external quality assessment scheme (DGKLRFB-Referenzinstitut für Bionalytik), the results of which were satisfactory in 100% of the cases.
Other measurementsWe collected data on diet and other variables such as time spent outdoors through personal interviews performed by specially trained interviewers when participants were 4 years old. The details of the overall INMA project can be found in a previous publication.19 The daily dietary intake of vitamin D was assessed with a semiquantitative food frequency questionnaire (FFQ) of each child's diet that was completed by the parents. This FFQ was similar to the one used by Willett et al. in the Nurse's Health Study of the United States,20 which has been adapted and validated for its use in the Spanish population, for instance in the INMA project.21,22 We also collected information on vitamin D supplementation (including multivitamin supplements). The validity of the FFQ in children was assessed in 169 children included in the study using three 24-hour dietary recalls and serum levels of different vitamins as the reference. In the assessment of reproducibility, the mean correlation coefficient was 0.42 for nutrients and 0.43 for foods. In the assessment of validity, the mean correlation coefficient for nutrient intake was 0.30 (results not published). Energy-adjusted intake was calculated by the residual method, regressing each nutrient on total calories and then adding back the population mean to the calculated residuals.20 Since vitamin D is transported in plasma lipoproteins, the levels of 25(OH)D3 were also adjusted per plasma cholesterol levels using the residual method.
Participating children underwent a physical examination with anthropometric measurements at age 4 years. Weight was measured on a scale accurate to 10g with the children barefoot and in their underwear. Height was measured with a calibrated wall-mount stadiometer on a millimetre scale. We calculated the body mass index (BMI) as weight (kg) divided by the squared height (m2). We classified children as having normal weight, overweight or obesity following the criteria of the International Obesity Task Force (IOTF) (Cole et al., 2000).23
Statistical analysisWe performed a descriptive analysis of the population under study, studying its sociodemographic and lifestyle characteristics in relation to 25(OH)D3 levels.
We used Pearson's correlation coefficient to analyse the correlation between serum levels of 25(OH)D3 and other continuous variables potentially associated with these levels. We also analysed partial correlations controlling for serum levels of cholesterol.
We used the χ2 test to compare categorical variables and ANOVA to compare the mean levels of 25(OH)D3 for different categories.
We conducted stepwise multivariate regression with backward elimination, including all the potential associated factors in the first step, with an entry criterion of 0.5 and a removal criterion of 0.10.
We set the level of statistical significance at an α of 0.05, and conducted the statistical analyses with SPSS version 15.0.
ResultsOf all patients, 52.3% were male, 94% were born to term, most were of Spanish descent (96.5%), and all were Caucasian except for two that were biracial. Also, 8.5% of the patients were obese and 13.4% overweight. None had kidney, parathyroid, liver or malabsorption disorders. Table 1 summarises the characteristics of the children in the INMA-Asturias cohort.
Characteristics of children in the Asturias cohort of the INMA project at age 4 years.
| Serum levels of 25(OH)D3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | <20 | 20–29.9 | ≥30 | P (χ2) | |||||
| n | % | n | % | n | % | n | % | ||
| Sex | |||||||||
| Female | 135 | 47.7 | 77 | 57.0 | 46 | 34.1 | 12 | 8.9 | .322 |
| Male | 148 | 52.3 | 72 | 48.6 | 63 | 42.6 | 13 | 8.8 | |
| Preterm birth (<37 weeks’ gestation) | |||||||||
| No | 263 | 94.0 | 143 | 54.4 | 98 | 37.3 | 22 | 8.4 | .042 |
| Yes | 17 | 6.0 | 4 | 23.5 | 10 | 58.8 | 3 | 17.6 | |
| BMI | |||||||||
| Normal | 218 | 77.9 | 120 | 55.0 | 79 | 36.2 | 19 | 8.7 | .585 |
| Overweight | 38 | 13.6 | 17 | 44.7 | 17 | 44.7 | 4 | 10.5 | |
| Obesity | 24 | 8.6 | 10 | 41.7 | 12 | 50.0 | 2 | 8.3 | |
| Missing values | 3 | ||||||||
| Overweight/obese | |||||||||
| No | 218 | 77.9 | 120 | 55.0 | 79 | 36.2 | 19 | 8.7 | .265 |
| Yes | 62 | 22.1 | 27 | 43.5 | 29 | 46.8 | 6 | 9.7 | |
| Season of the year | |||||||||
| Winter | 85 | 30.2 | 61 | 71.8 | 22 | 25.9 | 2 | 2.4 | <.001 |
| Spring | 80 | 28.5 | 43 | 53.8 | 33 | 41.3 | 4 | 5.0 | |
| Summer | 29 | 10.3 | 10 | 34.5 | 16 | 55.2 | 3 | 10.3 | |
| Autumn | 87 | 31.0 | 33 | 37.9 | 38 | 43.7 | 16 | 18.4 | |
| Missing values | 2 | ||||||||
| Month of sample collection | |||||||||
| June–September | 46 | 16.4 | 14 | 30.4 | 28 | 60.9 | 4 | 8.7 | .003 |
| Rest of the year | 235 | 83.6 | 133 | 56.6 | 81 | 34.5 | 21 | 8.9 | |
| Hours/day spent outdoors | |||||||||
| ≤ 1h | 8 | 4.6 | 4 | 4.7 | 3 | 4.3 | 1 | 5.0 | .988 |
| >1h | 167 | 95.4 | 81 | 95.3 | 67 | 95.7 | 19 | 95.0 | |
| Missing values | 108 | ||||||||
| Energy-adjusted vitamin D intake (ng/mL) | |||||||||
| < 5 | 248 | 89.9 | 132 | 53.2 | 94 | 37.9 | 22 | 8.9 | .677 |
| 5–10 | 28 | 10.1 | 13 | 46.4 | 13 | 46.4 | 2 | 7.1 | |
| > 10 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Missing values | 7 | ||||||||
| Vitamin D at 12 weeks’ gestation (ng/mL) | |||||||||
| < 20 | 74 | 27.8 | 50 | 67.6 | 20 | 27.0 | 4 | 5.4 | .007 |
| 20–30 | 92 | 34.6 | 45 | 48.9 | 43 | 46.7 | 4 | 4.3 | |
| ≥ 30 | 100 | 37.6 | 45 | 45.0 | 42 | 42.0 | 13 | 13.0 | |
| Missing values | 17 | ||||||||
| Maternal social class | |||||||||
| I+II | 68 | 24.1 | 33 | 48.5 | 28 | 41.2 | 7 | 10.3 | .239 |
| III | 55 | 19.5 | 24 | 43.6 | 23 | 41.8 | 8 | 14.5 | |
| IV+V | 159 | 56.4 | 91 | 57.2 | 58 | 36.5 | 10 | 6.3 | |
| Missing values | 1 | ||||||||
| Maternal country of origin | |||||||||
| Spain | 272 | 96.1 | 143 | 52.6 | 104 | 38.2 | 25 | 9.2 | .559 |
| Other | 11 | 3.9 | 6 | 54.5 | 5 | 45.5 | 0 | 0.0 | |
Statistically significant values shown in boldface.
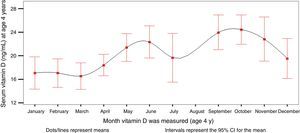
The mean serum level of 25(OH)D3 was 20.14ng/mL (range, 2.7–49.8). Of all participants, 8.8% had 25(OH)D3 levels of 30ng/mL or greater, 38.5% levels between 20 and 29.9ng/mL, and 52.7% levels of less than 20ng/mL. Serum levels varied based on the month that the blood sample was drawn (Fig. 1, Tables 1–4). When we grouped the results by season of the year, we observed that the levels of 25(OH)D3 were lower in the winter.
Distribution of serum levels of 25(OH)D3 at age 4 years in children in the Asturias cohort of the INMA project, showing percentiles and seasonal variation, and levels of intact parathormone (normal range [NR], 15–65), phosphate (NR, 0.95–1.65) and calcium (NR, 2.25–2.75).
| n | Mean | Minimum | Q1 | Median | Q3 | Maximum | |
|---|---|---|---|---|---|---|---|
| 25(OH)D3 (ng/mL) | |||||||
| January | 31 | 17.08 | 2.77 | 10.32 | 17.75 | 24.22 | 32.36 |
| February | 35 | 17.07 | 5.41 | 12.82 | 16.58 | 22.92 | 38.81 |
| March | 24 | 16.56 | 7.68 | 12.72 | 17.08 | 20.51 | 27.64 |
| April | 30 | 18.42 | 9.02 | 15.03 | 17.56 | 20.37 | 29.50 |
| May | 31 | 21.41 | 10.83 | 15.79 | 22.51 | 27.08 | 33.02 |
| June | 17 | 22.34 | 7.16 | 21.55 | 23.11 | 25.01 | 29.66 |
| July | 14 | 19.67 | 10.49 | 15.63 | 18.78 | 21.57 | 34.16 |
| August | 0 | . | . | . | . | . | . |
| September | 15 | 23.98 | 15.49 | 19.77 | 23.57 | 28.84 | 31.13 |
| October | 34 | 24.46 | 12.14 | 19.97 | 22.90 | 29.90 | 40.19 |
| November | 29 | 22.85 | 7.26 | 15.05 | 24.44 | 28.16 | 49.80 |
| December | 21 | 19.55 | 5.80 | 15.68 | 17.94 | 23.48 | 37.61 |
| Total | 281 | 20.14 | 2.77 | 15.49 | 19.78 | 24.56 | 49.80 |
| Intact parahormone (pg/mL) (NR, 15–65) | 58 | 35.15 | 15.07 | 27.22 | 33.71 | 42.00 | 76.40 |
| Phosphate (mmol/L) (NR, 0.95–1.65) | 181 | 1.62 | 1.26 | 1.54 | 1.63 | 1.71 | 2.02 |
| Calcium (mmol/L) (NR, 2.25–2.75) | 181 | 2.44 | 2.22 | 2.38 | 2.43 | 2.49 | 2.79 |
Factors associated to serum levels of vitamin D in the Asturias cohort of the INMA project at age 4 years.
| Serum vitamin D (ng/mL) in children at age 4 years (continuous scale) | |||||
|---|---|---|---|---|---|
| β | SD | 95% CI (β) | Partial correlation2 | ||
| Constant | 2.471 | 6.014 | −9.434 | 14.376 | |
| Month vitamin D analysed (4 y) | 0.497 | 0.166 | 0.168 | 0.826 | 0.261 |
| BMI at age 4 y | 0.714 | 0.350 | 0.021 | 1.408 | 0.181 |
| Maternal serum vitamin D (ng/mL) at 12 weeks’ gestation | 0.111 | 0.060 | −0.009 | 0.230 | 0.164 |
Factors associated to vitamin D deficiency/insufficiency assessed by serum levels in the Asturias cohort of the INMA project at age 4 years.
| Vitamin D deficiency/insufficiency by serum levels (ng/mL), age 4 years | ||||
|---|---|---|---|---|
| β | OR | IC (OR) del 95% | ||
| Constant | 1.360 | 3.897 | ||
| Mean time spent outdoors, age 4 years | 0.000 | 1.000 | 1.000 | 1.000 |
| Month vitamin D level, age 4 years | 0.220 | 1.246 | 1.038 | 1.496 |
| Total energy intake, age 4 years | −0.002 | 0.998 | 0.995 | 1.000 |
All the children that had severe vitamin D deficiency (n=21; 25-hydroxyvitamin D3<10ng/mL) had their blood samples drawn in seasons other than summer except for one, and the mothers of 64.7% of these children had vitamin D deficiency or insufficiency.
The mean time spent outdoors was three hours (range, 0:21–6:55), with all children exceeding the recommended minimum. We found no association between time outdoors and 25(OH)D3 levels (Table 4).
We calculated vitamin D intake (Table 5) from the estimated dietary intake, and found a median of 2.7μg/day (range, 0.81–12.62). The main dietary sources of vitamin D were fortified cereals and dairy products, followed by eggs and blue fish (Table 6). We did not find an association between serum levels and total vitamin D intake (Pearson correlation coefficient, 0.109; P=0.070) nor a partial correlation controlling for energy intake (Pearson correlation coefficient, 0.112; P=.062) or cholesterol levels. We also did not find a correlation when we grouped different foods (fish, white and blue fish, eggs, dairy) or adjusted for season or energy intake. None of the children were currently taking vitamin D supplements.
Ten foods that contribute most to vitamin D intake in children in the Asturias cohort of the INMA project at age 4 years (n=283).
| % Contribution to vitamin D intake | |
|---|---|
| Breakfast cereals (sweetened/chocolate) | 9.71 |
| Whole milk (alone, with powdered cocoa, or added to cereal) | 8.73 |
| Special growth and development fortified milks | 8.46 |
| Other fortified milks: vitamins A+D, folate and others, including those with added cereal | 7.90 |
| Chicken eggs: fried, scrambled, omelette, etc. | 7.88 |
| Swordfish | 6.68 |
| Large or medium-sized blue fish, such as tuna, bonito, salmon | 6.22 |
| Milkshakes such as Cacaolat, Colacao Energy, and others | 5.86 |
| Pan-grilled or boiled white fish | 5.53 |
| Canned tuna, sardines or mackerel | 4.08 |
We did not find an association between overweight and/or obesity and 25(OH)D3 levels (Table 3), nor an increased proportion of vitamin D deficiency/insufficiency in children with excess weight.
There were no statistically significant differences in 25(OH)D3 levels between the sexes (Table 1).
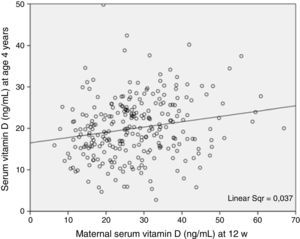
On the other hand, 25(OH)D3 levels in children aged 4 years were correlated to maternal levels at 12 weeks’ gestation, an association that was statistically significant when compared in terms of numerical values on a continuous scale and in terms of vitamin D categories—deficiency, insufficiency and sufficiency (Tables 1–3; Fig. 2).
DiscussionThe worldwide prevalence of vitamin D insufficiency/deficiency is estimated at approximately 100 million people.24 Furthermore, there is evidence of a decreasing trend in vitamin D levels worldwide in the past 10–20 years.25 Although many studies have been published on this subject, we do not know the prevalence of hypovitaminosis D in prepubertal children in Spain. In this population-based cohort study, we analysed serum levels of 25(OH)D3, vitamin D sources (dietary intake of vitamin D and sunlight exposure) and other associated factors in prepubertal Spanish children, and found a high prevalence of insufficient and deficient vitamin D levels (20–20.9ng/mL in 38.5%, and <20ng/mL in 52.7%). The main strength of this study is that it was carried out in a cohort of healthy children that have been followed up from the prenatal period. Also, we controlled for potential risk factors, and their births were distributed throughout the year.
A recent study conducted in Cadiz in 146 children aged 10–14 years found levels of less than 20ng/mL in 45.2%, a prevalence that was slightly lower than the prevalence we found at age 4 years, but especially lower when we compared it with our results for blood samples collected in March (the month when the Cadiz study was conducted): 75th percentile, 20.51ng/mL. The difference was even greater when we compared our results to those of another study conducted in prepubertal girls aged 7–10 years in Cordoba, which found a mean level of 40.07ng/mL with a standard deviation of 10.49, no participants with levels below 20ng/mL, and levels between 20 and 30ng/mL in 25%.27 On the other hand, a study conducted in Catalonia only found 25(OH)D3 levels of less than 20ng/mL in 8% of the 85 Caucasian children (infants and children aged <5 year).28 This difference could be due to lower exposure to sunlight in children in our region on account of its latitude (43°) and greater degree of cloudiness, along with the potential impact of age and lifestyle. Another factor that may contribute to these differences is the smaller sample size of these other studies compared to ours. A study conducted in 413 children and adolescents aged 3 to 15 years in Navarra, a region with a similar latitude (43°–41°), found higher levels of 25(OH)D3 (sufficiency in 42.4%, insufficiency in 44.9% and deficiency in 12.7%),29 which could be attributed to differences in cloudiness, as there are fewer hours of sunlight in Asturias, as well as differences in age and methodology (determination of 25-hydroxyvitamin D by chemiluminescence).
On the other hand, a study conducted in infants in Asturias found no evidence to support routine supplementation in this age group, although the authors underscored the importance of strictly monitoring exclusively breastfed infants, especially in the winter and in the early months of life.30 Our findings suggest that supplementation may be necessary after infancy to prevent the low levels found at age 4 years.
In most people, the main source of vitamin D is moderate sun exposure, so it follows that insufficient exposure is the leading cause of vitamin D deficiency. There is published evidence of seasonal variations in 25(OH)D3 levels,1 which our results corroborate. The slight decline in July may be insignificant considering the lower number of children tested in this month due to summer holidays and an increased variability in the results. Seasonal variations in bone turnover in prepubertal children and early puberty has also been described in the literature.31 We did not find an association between time spent outdoors and 25(OH)D3 levels, although all children exceeded the recommended 15min. This could be explained in part by factors that reduce the effectiveness of sunlight exposure, such as the increased coverage with clothing in the cold months, the cloudy weather, the increased use of sunscreen, the times of day the children are outdoors and the latitude in Asturias. Thus, the general recommendation of 15min of sunlight exposure a day may not suffice to guarantee optimal levels of 25(OH)D3 in locations at this latitude. In order to ensure an effective exposure, there should be an emphasis on recommending a minimum of 10–15min in the sun without protection, at least in the arms and legs, in the spring, summer and autumn.
The second major source of vitamin D is diet. At present, the Committee on Nutrition of the American Academy of Paediatrics (AAP) and the IOM agree on increasing the recommended daily vitamin D allowance for children aged 1–18 years to 600vIU/day (15μg/day), which is also supported by the Committee on Nutrition of the Spanish Association of Paediatrics (AEP).17,32 In the Endocrine Society clinical practise guideline (2011), Holick et al. recommend a minimum dietary intake of 600IU/day, although intakes of up to 1000IU/day may be needed.16 None of the children in our study had intakes that met the currently recommended minimum of 15μg/day, and 75% did not even reach 5μg/day, revealing a significantly deficient intake. These extremely low intakes, lower even than those of the mothers, may be the reason we did not find an association between intake and serum 25(OH)D3 levels.
We did not find any differences between the sexes in our cohort, although studies with prepubertal participants show an incipient sexual dimorphism.26,29
We did not find an association between BMI and 25(OH)D3 levels, which was not consistent with the findings of other studies conducted in children that showed a correlation between obesity and a higher prevalence of vitamin D deficiency. This was the case in the 2003–2006 National Health and Nutrition Examination Survey (NHANES), which studied 12,292 children aged 6–18 years.33 The difference may be due to the fact that this association was strongest in severely obese children, of whom there were none in our cohort, as well as the difference in the ages of the participants. On the other hand, the overall analysis of all the INMA cohorts found an association between deficient 25(OH)D3 levels in pregnancy (n=2644) and an increased risk of overweight and obesity in children at age 1 year (OR=1.42; 95% CI, 1.02–1.97), although this association was weaker for age 4 years (OR=1.19; 95% CI, 0.83–1.72). We ought to note that this study used the WHO growth standards as reference.34
None of the children in our cohort were taking vitamin D supplements, despite the high prevalence of vitamin D deficiency/insufficiency and the low dietary intake, both of which were more marked in children at age 4 years than in their mothers during pregnancy, as we previously reported.35 The indications for supplementation in children are still under debate. There is evidence that supplementation is more effective in achieving adequate levels of vitamin D than nutritional education interventions specifically designed to prevent vitamin D deficiency.36 The appropriate dose for supplementation is also under debate. A recent study in children conducted in Pennsylvania (latitude, 40.4°) between October and March with supplementation with 1000IU/day found that while this dose achieved an increase in serum levels, it was not sufficient to remedy vitamin D deficiency.37
Our findings support the promotion of a varied diet with an adequate intake of foods rich in vitamin D such as eggs, blue fish and fortified foods, especially in the winter, this age group, and in our region, as well as considering supplementation in children with risk factors for vitamin D deficiency or insufficiency.
At present, routine screening of the paediatric population for vitamin D deficiency is not recommended, but it should be considered in patients with known risk factors, such as exclusively breastfed babies that do not receive supplementation, dark-skinned children and adolescents residing in northern countries, children and adolescents with inadequate sunlight exposure (excessive use of sunscreens with high SPF, that spent most of the day indoors, that use clothing covering most of the skin, during the winter in northern latitudes etc.) or with obesity.38 Screening would also be warranted in the following situations: clinical suspicion of rickets, genu varum, abnormal fractures, bone pain, hypocalcaemia or hypophosphataemia, chronic liver or kidney disease, malabsorption, severe malnutrition, lack of sun exposure, and treatment with anticonvulsants, growth hormone, glucocorticoids or antiretrovirals.39
The association between 25(OH)D3 levels during gestation and at age 4 years leads us to believe that deficiency of insufficiency in these cases is maintained throughout this time interval and associated with dietary and lifestyle habits. On the other hand, the fact that insufficiency and deficiency were more prevalent in these children compared to their mothers during pregnancy highlights the need to provide information and implement health education measures to promote improved levels in children, as is done in relation to pregnancy.
In conclusion, we found a high prevalence of vitamin D deficiency and insufficiency at age 4 years, especially in the winter. The intake of vitamin D from dietary sources is generally insufficient, so we need health promotion strategies with an emphasis on adequate intake of foods rich in vitamin D and to consider supplementation in children of this age with known risk factors. Although children did spend the recommended time outdoors, this was not associated with their serum 25(OH)D3 levels. Therefore, we need to promote effective exposure to sunlight in children residing in Asturias. We found an association between serum 25(OH)D3 levels during gestation and at age 4 years, which suggests that the deficiency/insufficiency is maintained throughout this time span. We must make this known both to the general population and to health care professionals, given the importance of maintaining an adequate vitamin D status.
FundingGrants for research projects on health of the Instituto de Salud Carlos III. Ministry of Science and Innovation. European Regional Development Fund (ERDF) projects PI042018, PI0902311, and PI1302429.
Grants for research projects on health of the Instituto de Salud Carlos III. Ministry of Science and Innovation. ERDF projects PI070314 and PI11/01007.
Conflict of interestsThe authors have no conflict of interests to declare.
We thank all the families in the Asturias cohort of the INMA project for their disinterested participation in the project, without who this work would not have been possible. We also thank the rest of the team that works with the Asturias INMA cohort.
Please cite this article as: Rodríguez-Dehli AC, Riaño-Galán IR, Fernández-Somoano A, Navarrete-Muñoz EM, Espada M, Vioque J, et al. Hipovitaminosis D y factores asociados a los 4 años en el norte de España. An Pediatr (Barc). 2017;86:188–196.