The health-related quality of life (HRQoL) questionnaire is important in order to assess the effects of therapeutic intervention. The aim of this study is to analyse HRQoL, comparing cases of attention deficit hyperactivity disorder (ADHD) treated with methylphenidate (ADHD-T), untreated cases (ADHD-N), and controls.
Material and methodsThe study included a sample of 228 participants between 8 and 14 years old (114 controls, 57 ADHD-T, and 57 ADHD-N). Consecutive sampling was used in ADHD according to DSM-IV criteria (ADHD Rating Scales IV), and random sampling of controls matched by gender and age. The evaluation of HRQoL was made by using KIDSCREEN-52 parent version.
ResultsThe intensity of ADHD symptoms is significantly lower in ADHD-T than in ADHD-N. There is a moderate significant correlation between greater intensity of ADHD symptoms and worse HRQoL. ADHD cases have significantly worse HRQoL than controls on psychic well-being, mood, relationship with parents and friends, school environment, and social acceptance. The cases of ADHD-T have significantly better HRQoL than ADHD-N in the school dimension, but do not differ significantly in other dimensions of KIDSCREEN-52.
ConclusionsIt would be advisable that the treatment of ADHD integrates multi-dimensional therapeutic models that improve the basic symptoms of the disorder, as well as the HRQoL.
La calidad de vida relacionada con la salud (CVRS) es un marcador relevante para valorar los efectos de una intervención terapéutica. El objetivo del estudio es analizar la CVRS comparando casos con trastorno por déficit de atención con hiperactividad (TDAH) tratados farmacológicamente con metilfenidato (TDAH-T), casos no tratados (TDAH-N) y controles.
Material y métodosMuestra de 228 participantes entre 8 y 14 años (114 controles, 57 TDAH-T y 57 TDAH-N). Muestreo consecutivo de TDAH según DSM-IV (ADHD Rating Scales IV) y muestreo aleatorio de controles emparejados por sexo y edad. Evaluación de CVRS mediante KIDSCREEN-52 versión padres.
ResultadosLa intensidad de síntomas de TDAH es significativamente menor en TDAH-T que en TDAH-N y se observa correlación significativa moderada entre mayor intensidad de síntomas de TDAH y peor CVRS. Los casos de TDAH tienen significativamente peor CVRS que los controles en bienestar psíquico, estado de ánimo, relación con padres, relación con amigos, entorno escolar y aceptación social. Los casos de TDAH-T presentan significativamente mejor CVRS que TDAH-N en la dimensión escolar, pero no se diferencian significativamente en otras dimensiones del KIDSCREEN-52.
ConclusionesSería recomendable que el tratamiento del TDAH integrase modelos terapéuticos multidimensionales que mejoren los síntomas básicos del trastorno y la CVRS.
Our study focuses on the multidimensional analysis of quality of life in patients with attention-deficit hyperactivity disorder (ADHD) receiving or not receiving medication and their comparison with controls matched for sex, age and area socioeconomic status.
Attention-deficit hyperactivity disorder is characterised by a persistent pattern of inattention and/or hyperactivity-impulsivity that interferes with functioning or development, present before 12 years in 2 or more settings and with an impact on social, school or work functioning.1
From an epidemiological perspective, a systematic review of the prevalence of ADHD in children and adolescents worldwide revealed a global average prevalence of 5.29%2 and a prevalence of 6.6%3 in Spain, stable throughout age groups (6.9% in children, 6.2% in preadolescents and 6.9% in adolescents).4
Attention-deficit hyperactivity disorder generates an increasing demand for mental health services and is associated with more symptoms, family problems and school problems compared to the general population and/or clinical controls,5 with evidence of poorer long-term outcomes in academic performance, job performance, high-risk sexual behaviours and early unwanted pregnancies, substance use, relationship difficulties, marital problems, traffic violations, and car accidents,6 all of which makes early diagnosis and intervention particularly important.
At present, ADHD is mainly a clinical diagnosis based on DSM-5 or ICD-10 criteria, requiring documentation of clinically significant impairment in social, school or work functioning and how the symptoms impact quality of life. On the other hand, clinical trials, in addition to analysing the efficacy of treatment on relieving the symptoms of ADHD, have been increasingly emphasising the importance of assessing the impact on quality of life.
Health-related quality of life (HRQoL), which interprets health as something greater than the absence of disease,7 is a multidimensional construct based on indicators of physical, psychological, social and cognitive functioning that is measured with instruments that include these domains in varying degrees.8,9
The existing systematic reviews and meta-analyses evince an impact on different domains of HRQoL in children with ADHD compared to controls,9 describing a poorer HRQoL in the psychological,10 psychosocial11,12 and academic10 domains, with a lesser impact on physical measures.11,12
Several authors have reported that the use of medication to treat ADHD can improve13,14 or have an impact on quality of life,15–17 while other authors have found no differences between patients with ADHD based on whether they did nor did not receive pharmacological treatment.9
In short, patients with ADHD usually exhibit a poorer HRQoL compared to controls, although not in every domain and with variability between studies. The evidence on the impact of pharmacological treatment on HRQoL in patients with ADHD is also inconsistent. The heterogeneity of the findings may be partly explained by the use of different HRQoL questionnaires and differences in sample size or selection criteria or the study design. At any rate, understanding the impact of ADHD on quality of life is relevant to the functional assessment required for diagnosis, and HRQoL is an important and reliable indicator of the effect of therapeutic intervention, which should not be assessed solely based on symptom reduction.
The aim of the study presented here was to assess HRQoL through the broad range of dimensions included in the KIDSCREEN-52 questionnaire,18 comparing the results in children with ADHD treated with methylphenidate, children with ADHD without pharmacological treatment and controls.
This is the first study of its kind in the national and international scientific literature, as it compares, in a significant sample, cases of ADHD treated and not treated with medication and healthy controls using as large a set of dimensions of HRQoL as the one comprising the KIDSCREEN-52,18 which would allow for more precise differentiation in comparison tests.
Materials and methodsStudy designWe conducted an observational and analytical study. The participants were aged 8–14 years and included patients with a diagnosis of ADHD receiving pharmacological treatment with methylphenidate (ADHD-T), patients with ADHD not receiving pharmacological treatment (ADHD-N), and controls matched for sex, age and area socioeconomic status.
For a year, until we achieved the necessary sample size, we recruited new cases of ADHD-N by consecutive enrolment of patients that had their initial appointments at the primary care paediatrics and mental health clinics. Considering the method used to obtain this initial sample, we selected ADHD-T cases in the same context by consecutive sampling ensuring matching of ADHD-N cases for sex, age and area socioeconomic status.
We selected the controls in the paediatric primary care clinics by stratified randomised sampling, also ensuring matching of ADHD cases for sex, age and area socioeconomic status.
We defined the presence of ADHD based on a clinical interview adapted to the diagnostic criteria of the DSM-IV and a score above the 93rd percentile in the Attention-Deficit/Hyperactivity Disorder Rating Scales IV (ADHD RS-IV).19 Inclusion in the ADHD-T group required having received medication (methylphenidate) for at least 3 months, and inclusion in the ADHD-N group never having received medication. The inclusion criteria for controls were consent to participation and absence of ADHD based on the same criteria used to define the cases. We obtained written informed consent from the parents of all participants, and the study was reviewed and approved by the Research Board of the hospital. With this design and aiming to fulfil the primary objective of our study, we compared the responses to the proxy version completed by parents of the KIDSCREEN-5218 quality of life questionnaire in the 3 groups of participants.
When it came to data analysis, we used different descriptive and inferential statistics based on the distribution of the data of the variables under study. For any analysis of correlation or comparison of variables, we defined statistical significance as an α level of 0.05. We used the χ2 test to analyse the association or independence of nominal variables.
To assess the difference in the mean values of quality of life measures in the 3 groups under study, we used factorial analysis of variance (ANOVA). In multiple comparisons of the 3 factors (ADHD-T/ADHD-N and controls), we controlled the risk of type I error by means of the Bonferroni correction. In the factorial ANOVA, we used the η2 statistic to measure the size of the effect of the factors on quality of life.
We also used factorial ANOVA to assess differences in the mean scores in the ADHD RS-IV between the 3 groups.
We calculated the sample size required to detect a minimum relevant difference of 5 points in the KIDSCREEN-52 between the 3 types of participants, which was of 57 children per group. We calculate the size for an alpha error of 0.05 and a beta error of 0.2 in two-tailed tests assuming a standard deviation of 8.2.
ParticipantsThe sample included 228 participants aged 8–14 years, of who 114 were controls and 114 children with ADHD. The cases included 57 children in the ADHD-T group and 57 in the ADHD-N group. We recruited participants in primary care centres (in the cities of Palencia and Valladolid) and in a mental health clinic (Palencia). The results section provides a more detailed description of the characteristics of the sample.
Variables and instrumentsKIDSCREEN-52 questionnaire.18 It is an instrument used to measure HRQoL in children and adolescents aged 8–18 years that was developed simultaneously in 13 European countries (including Spain) to guarantee transcultural equivalence and its applicability to different populations. During the development process, the instrument adapted to the Spanish population exhibited adequate psychometric properties.20,21 Structural equation modelling and confirmatory factor analysis revealed that the KIDSCREEN-52 had a structure that included 10 dimensions and 52 items: physical wellbeing (5 items) assesses physical activity, energy and fitness; psychological wellbeing (6 items) includes items on positive emotions, satisfaction with life and perceived emotional balance; moods and emotions (7 items) covers negative experiences depressive moods and stressful feelings; self-perception (5 items) explores how respondents perceive their bodily appearance and body image, and their satisfaction with their looks; autonomy (5 items) examines self-sufficiency, independence and the opportunity to participate in social activities; parent relation and home life (6 items) assesses the relationship with the parents, the atmosphere in the household and whether the respondent feels he or she is of an appropriate age to become independent; peers and social support (6 items) examines the how the respondent tends to relate to other children or adolescents and the support received; school environment (6 items) explores how children/adolescents perceive their cognitive skills, learning and concentration and how they feel about school; social acceptance/bullying (3 items) covers the aspect of feeling accepted or intimidated by peers in school; and financial resources (3 items) assesses the degree of satisfaction of respondents with their financial resources and whether they allow matching the standard of living of peers.
The scores in each dimension are converted to t scores, with higher scores reflecting a better HRQoL.
The analysis of the psychometric properties of the KIDSCREEN questionnaire version that includes 52 items found an acceptable validity and reliability overall and for the Spanish version in particular.20 In the Spanish version, the internal consistency of the 10 dimensions of the KIDSCREEN assessed by means of Cronbach's alpha ranged from 0.74 to 0.88 for the different dimensions. The confirmatory factor analysis indicated that the 10-dimension structure of the questionnaire was a good fit for the data.20 As for validity, the KIDSCREEN-52 exhibited moderate to strong correlations with other quality of life questionnaires (KINDL, Youth-QoL and CHIP-AE) and a very good construct validity, both convergent and discriminant.20
In this study, we used the KIDSCREEN-52 proxy version completed by the parents because the subjective assessment of HRQoL through a 52-item questionnaire is complicated in children with ADHD by impaired concentration and self-control, which could hinder their ability to rate themselves accurately and complete the questionnaire correctly.9
ADHD RS-IV.19 This questionnaire is designed to match the criteria of the DSM-IV. Parents rate each item based on the frequency of the specific criterion in the past 6 months. Each item is rated on a frequency scale from 0 to 3 (never or rarely, sometimes, often and very often). The internal consistency of the ADHD RS-IV assessed by means of Cronbach's alpha was 0.94 and the test–retest reliability assessed by means of the Pearson correlation coefficient was 0.90. The questionnaire has exhibited an adequate correlation with other questionnaires commonly used in the assessment of ADHD, such as the Conners’ Teacher Rating Scale-39 (r=0.88) and the Conners’ Parent Rating Scale-48 (r=0.80). The criterion validity for ADHD reported in different studies ranges between 0.72 and 0.90.21
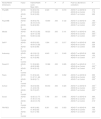
ResultsTable 1 summarises the descriptive data for the sample, in which 78.5% of participants were male and 21.5% female, with the same sex distribution in cases and controls. There were no significant ages in mean age between cases and controls (t=1.351; P=.178). As a result of the design, we also did not find significant differences between the types of participants in the sex distribution (χ2=0.000; P=1), or the mean age (F=0.000; P=1). The distribution of cases by ADHD type was 39% (n=45) inattentive type, 2.6% (n=3) hyperactive-impulsive type and 57.9% (n=66) combination type, and there were no significant differences in this distribution between the ADHD-T group and the ADHD-N group (χ2=3.103; P=.212).
Sample characteristics.
| Sex (n; %) | Group (n; %) | Age: mean (SD) |
|---|---|---|
| Male (179; 78.5) | ADHD-T=(44; 25) ADHD-N=(44; 25) Control=(88; 50) | 10.05 (1.91) 10.05 (1.91) 10.05 (1.92) |
| Female (49; 21.5) | ADHD-T=(13; 25) ADHD-N=(13; 25) Control=(26; 25) | 10.46 (2.14) 10.46 (2.14) 10.46 (2.10) |
| Total (228; 100) | ADHD-T=(57; 25) ADHD-N=(57; 25) Control=(114; 50) | 10.14 (1.96) 10.14 (1.96) 10.14 (1.95) |
ADHD, attention-deficit hyperactivity disorder; ADHD-N, ADHD with no treatment; ADHD-T, ADHD with pharmacological treatment; SD, standard deviation.
Table 2 shows the mean scores in the ADHD RS-IV in the 3 groups under study. We found significant differences between the control, ADHD-T and ADHD-N groups. These significant differences (after performing the Bonferroni correction) involved a greater severity of symptoms in the ADHD-N compared to the ADHD-T group, and in the ADHD-T group compared to controls. The effect size of medication in the comparison of the means of the ADHD-T and the ADHD-N groups was medium, although it was near the threshold for large (Cohen's d=0.745).
Mean scores in the ADHD RS-IV in patients with ADHD receiving medication, patients with ADHD not receiving medication and controls.
| Group | Mean (SD) | Snedecor's F | P | Significant differences in comparisons |
|---|---|---|---|---|
| ADHD-T (T) ADHD-N (T) Control (T) | 27.12 (11.65) 34.57 (8.64) 8.13 (6.73) | 197.63 | .000* | ADHD-N>ADHD-T>Control |
| ADHD-T (I) ADHD-N (I) Control (I) | 14.71 (6.38) 17.91 (4.51) 4.48 (4.03) | 204.53 | .000* | ADHD-N>ADHD-T>Control |
| ADHD-T (H) ADHD-N (H) Control (H) | 12.33 (7.10) 14.80 (6.53) 3.68 (3.43) | 92.28 | .000* | ADHD-N>ADHD-T>Control |
ADHD, attention-deficit hyperactivity disorder; ADHD-N, ADHD with no treatment; ADHD-T, ADHD with pharmacological treatment; ADHD RS-IV, Attention-Deficit/Hyperactivity Disorder Rating Scales IV; SD, standard deviation; (H), hyperactivity-impulsivity items in the ADHD RS-IV; I, inattention items in the ADHD RS-IV; T, total score in the ADHD RS-IV.
We found significant negative correlations (P<.01) between the total score of the ADHD RS-IV and every HRQoL dimension in the KIDSCREEN-52 (physical wellbeing, r=−0.176; psychological wellbeing, r=−0.354; moods, r=−0.460; self-perception, r=−0.167; autonomy, r=−0.198; parent relation r=−0.334; financial resources, r=−0.309; peers, r=−0.305; school, r=−0.554; bullying, r=−0.311). A higher intensity of ADHD symptoms was associated with a poorer quality of life. These data are not presented in the tables.
Table 3 presents the results of factorial ANOVA in the 3 groups of participants for each of the dimensions of the KIDSCREEN-52 proxy version completed by the parents. Patients with ADHD, whether or not they were receiving medication, had lower mean scores in every dimension of the KIDSCREEN-52 compared to controls.
Factorial ANOVA (type of participant) for the dimensions of the KIDSCREEN-52.
| KIDSCREEN dimensions | Factor | KIDSCREEN score Mean (SD) | F | P | η2 | Post hoc (Bonferroni correction) | P |
|---|---|---|---|---|---|---|---|
| PhysWB | ADHD-T ADHD-N Control | 50.38 (9.72) 47.53 (9.03) 50.40 (9.26) | 2.020 | .135 | 0.018 | ADHD-T vs ADHD-N ADHD-N vs Control ADHD-T vs Control | .312 .176 .999 |
| PsychWB | ADHD-T ADHD-N Control | 49.48 (8.76) 46.18 (10.91) 54.46 (8.95) | 15.690 | .000 | 0.122 | ADHD-T vs ADHD-N ADHD-N vs Control ADHD-T vs Control | .189 .000*** .004** |
| Moods | ADHD-T ADHD-N Control | 46.16 (12.26) 42.81 (12.39) 53.69 (12.23) | 18.523 | .000 | 0.141 | ADHD-T vs ADHD-N ADHD-N vs Control ADHD-T vs Control | .392 .000*** .000*** |
| Self-P | ADHD-T ADHD-N Control | 49.59 (9.30) 49.54 (10.28) 52.00 (8.99) | 1.904 | .151 | 0.017 | ADHD-T vs ADHD-N ADHD-N vs Control ADHD-T vs Control | .999 .328 .348 |
| Autonomy | ADHD-T ADHD-N Control | 48.19 (8.13) 49.21 (9.27) 52.10 (8.49) | 4.643 | .011 | 0.040 | ADHD-T vs ADHD-N ADHD-N vs Control ADHD-T vs Control | .999 .120 .017* |
| Parent-R | ADHD-T ADHD-N Control | 51.39 (8.90) 49.50 (8.98) 55.42 (8.12) | 10.386 | .000 | 0.085 | ADHD-T vs ADHD-N ADHD-N vs Control ADHD-T vs Control | .717 .000*** .012* |
| Peers | ADHD-T ADHD-N Control | 51.83 (8.43) 51.87 (11.48) 56.68 (8.71) | 7.457 | .001 | 0.062 | ADHD-T vs ADHD-N ADHD-N vs Control ADHD-T vs Control | .999 .006** .005** |
| School | ADHD-T ADHD-N Control | 49.24 (8.08) 41.44 (9.18) 56.72 (9.67) | 54.043 | .000 | 0.324 | ADHD-T vs ADHD-N ADHD-N vs Control ADHD-T vs Control | .000*** .000*** .000*** |
| Social | ADHD-T ADHD-N Control | 42.92 (12.77) 41.50 (13.10) 48.34 (10.76) | 7.766 | .001 | 0.065 | ADHD-T vs ADHD-N ADHD-N vs Control ADHD-T vs Control | .999 .001** .016* |
| FIN-RES | ADHD-T ADHD-N Control | 49.50 (9.90) 51.48 (9.35) 54.31 (7.56) | 6.301 | .002 | 0.053 | ADHD-T vs ADHD-N ADHD-N vs Control ADHD-T vs Control | .668 .135 .002** |
ADHD-N, ADHD with no treatment; ADHD-T, ADHD with pharmacological treatment; F, Snedecor's F; FIN-RES, financial resources; Parent-R, parent relations and family life; PhysWB, physical wellbeing; PsychWB, psychological wellbeing; SD, standard deviation; Self-P, self-perception; Social, social acceptance/bullying.
The comparison of mean values in the ADHD-T and control groups revealed significant differences, with evidence of a poorer quality of life in patients in the ADHD-T group in the KIDSCREEN-52 dimensions of psychological wellbeing, mood, autonomy, parent relation, peers, school environment, bullying and financial resources. We found no differences in physical wellbeing and self-perception. The comparison of the ADHD-N and control groups was similar, except for the absence of significant differences in autonomy and financial resources dimensions of the KIDSCREEN-52.
The comparison of the ADHD-T and ADHD-N groups did not reveal significant differences in most dimensions of the KIDSCREEN-52, the exception being the school environment dimension, in which patients with ADHD-T exhibited better quality of life.
We performed an analysis of covariance taking into account the potential confounders of sex, age and ADHD type, and the results did not differ from those obtained in the previous analyses.
DiscussionPatients in the ADHD-N group had more severe inattention and hyperactivity/impulsivity symptoms compared to their ADHD-T counterparts, and the latter had more severe symptoms compared to controls. This finding was consistent with the reports on the short- and medium-term efficacy of pharmacological treatment for the reduction of the main symptoms of ADHD from randomised controlled trials.22,23 We also found that a greater severity of ADHD symptoms correlated to a poorer quality of life in every dimension of the KIDSCREEN-52, as has already been reported by systematic reviews analysing the association of ADHD and quality of life through different questionnaires completed by parents.6,9,24 A significant moderate correlation like the one found in our study supports the hypothesis that HRQoL and ADHD features are associated yet not the same, so that both need to be assessed to provide a complete picture of the difficulties of the child or adolescent with ADHD.9 Based on the results of our study, inattention and hyperactive/impulsive symptoms of ADHD seem to correlate to the same degree with the reduction in HRQoL.9
In the analysis performed in our study, controls generally exhibited a significantly greater quality of life compared to patients with ADHD in most dimensions of the KIDSCREEN-52. The only dimensions in which we did not find significant differences between the controls and patients in the ADHD-T or ADHD-N groups were physical wellbeing and self-perception, and, in the case of the ADHD-N group, autonomy and financial resources as well. We found the largest effect sizes in the differences in HRQoL between participants in relation to the school environment, psychological wellbeing and mood.
To our knowledge, no studies have been published to date with designs similar to ours, comparing groups of participants and using the multidimensional KIDSCREEN-52 questionnaire. Therefore, the discussion that follows will focus on studies that have assessed the association of ADHD and quality of life based on other questionnaires that include fewer dimensions, some of which coincide with the dimensions included in the KIDSCREEN-52.
The systematic reviews and meta-analyses on HRQoL in the context of ADHD have found that different domains of HRQoL are more impacted in individuals with ADHD compared to controls,9,12,13,24 and several authors have highlighted that children with ADHD exhibit a poorer quality of life in psychological,10 psychosocial11,12 and school-related10 variables, with lesser or no differences in physical health variables,9,11,12 as was the case in our study.
In our analysis, we found the largest effect of ADHD on quality of life in the school environment, psychological wellbeing and mood dimensions. Other studies have also found the largest effect in the dimensions of school environment,10,12 psychological wellbeing10 and mood.12
Our study did not find significant differences between ADHD cases and controls in self-perceived looks and body image, an aspect on which other studies have not reported results based on the use of the KIDSCREEN-52 questionnaire. Along the same lines, we did not find differences between the ADHD-N and the control groups in autonomy or perceived self-sufficiency, independence and opportunity to participate in social activities, nor in the degree of satisfaction with the available financial resources allowing a lifestyle comparable to that of peers.
When it came to the dimensions associated with pharmacological treatment with methylphenidate, patients in the ADHD-T group scored better in the school environment dimension compared to patients in the ADHD-N group, but we found no differences in any of the other dimensions of HRQoL included in the KIDSCREEN-52 questionnaire (physical wellbeing, psychological wellbeing, moods and emotions, self-perception, autonomy, parent relation, financial resources, peers or bullying), on which methylphenidate seemed to not have a significant effect.
There are other systematic reviews that used questionnaires other than the KIDSCREEN-52 that also did not find significant differences in quality of life between patients with ADHD treated pharmacologically and patients with ADHD that were not receiving medication.9
However, 3 clinical trials conducted by different groups that used measures of quality of life other than the KIDSCREEN-52 reported a generalised small beneficial effect of methylphenidate in quality of life.15–17
In short, our study reflected that individuals with ADHD generally had a poorer quality of life compared to controls, especially in dimensions related to school, psychological wellbeing and mood. We also found that treatment with methylphenidate improved the school dimension in the KIDSCREEN-52 questionnaire but had no significant effect in the other dimensions.
Attention-deficit hyperactivity disorder is a risk factor for a poor HRQoL, which underscores the need for early diagnosis and treatment in this disorder, implementing therapeutic interventions that address the affected dimensions and functional impairments.
Lastly, our study demonstrates that medication with methylphenidate improves ADHD symptoms and the school-related dimensions in the KIDSCREEN-52 but does not seem to have a significant impact on other dimensions, which suggests that pharmacological treatment should be provided as part of a multidimensional, holistic and multidisciplinary care plan25–27 that includes the improvement of quality of life among its goals.
In our opinion it is advisable that providers involved in the management of individuals with ADHD include the assessment of HRQoL and interventions for its improvement in their therapeutic armamentarium.
FundingThe study was funded by the Fundacion Ernesto Sánchez Villares (Project 05/2014) of the Sociedad de Pediatría de Asturias, Cantabria, Castilla y León (Project 05/2014).
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: López-Villalobos JA, Sacristán-Martín AM, Garrido-Redondo M, Martínez-Rivera MT, López-Sánchez MV, Rodríguez-Molinero L, et al. Calidad de vida relacionada con la salud en casos de trastorno por déficit de atención con hiperactividad con/sin tratamiento farmacológico. An Pediatr (Barc). 2019;90:272–279.