The aetiological agent of erythema infectiosum is Erythrovirus B19 (also known as parvovirus B19), frequently found in children and adolescents, but also associated with arthropathy, aplastic crisis, and abortion in adults.
Material and methodsA retrospective study of Erythrovirus B19 cases in the years 2010–2015.
ResultsOf the 56 cases of Erythrovirus B19 diagnosed, 34 were adults (32 women and 2 men) and 22 younger than 18 years (12 girls and 10 boys). Six cases were in pregnant women. Infections mainly occurred between spring and summer. In childhood, fever (64%), rash (50%), and anaemia (55%) were the most frequent symptoms. However, arthralgia (59%) was the most frequent symptom in adults, and less frequent were anaemia (41%), fever (32%), and rash (29%).
ConclusionsThe characteristic clinical presentation in childhood was rash and fever, whereas in adults it was arthralgia. Anaemia is also frequent, but only severe in previous haematological disease. It should be pointed out that Erythrovirus B19 infection during pregnancy could severely affect the foetus.
El Erythrovirus B19 (anteriormente denominado parvovirus B19) es el agente etiológico del eritema infeccioso que afecta mayoritariamente durante la infancia y la adolescencia, pero también está relacionado con artropatías, crisis aplásicas y abortos en adultos. El propósito de esta revisión es estudiar las características de las infecciones causadas por Erythrovirus B19 diagnosticadas en nuestro hospital en los últimos 6 años y las diferencias entre la población adulta y la pediátrica.
Material y métodosEstudio retrospectivo de los casos diagnosticados de Erythrovirus B19 mediante serología, entre enero de 2010 y diciembre de 2015.
ResultadosFueron diagnosticados 56 casos, 34 adultos (32 mujeres y 2 varones) y 22 menores de 18 años (12 niñas y 10 niños). El 75% de los casos se dieron entre primavera y verano. Seis fueron en gestantes y en 2 hubo complicaciones graves que conllevaron la muerte fetal. En la población pediátrica los síntomas más frecuentes fueron fiebre (64%), exantema (50%) y anemia (55%). En adultos las artralgias (59%) y menos frecuentemente la anemia (41%), la fiebre (32%) y el exantema (29%).
ConclusionesEn pediatría la clínica más frecuente es el exantema y la fiebre, y en adultos las artralgias. También es frecuente la anemia, los casos más graves en presencia de enfermedad hematológica previa. Hay que destacar la grave afectación que pueden sufrir los fetos en las gestantes.
Parvovirus B19 was first identified in 1974 by Cossart, and it was associated with disease for the first time in 1981 when it was detected in association with aplastic crises in patients with sickle cell disease.1,2 Due to its tropism for human erythroid progenitor cells, it was eventually reclassified as Erythrovirus B19 (B19V).1
Erythrovirus B19 is a small DNA virus whose only known host is Homo sapiens. It is mainly transmitted through respiratory secretions or hand-to-mouth contact,2 although vertical transmission from mother to foetus and iatrogenic transmission through blood products are also possible.1 Cases of nosocomial transmission have also been documented. Since it lacks a lipid envelope, it is resistant to physical inactivation with heat or detergents.3
This virus causes fifth disease or erythema infectiosum (EI) of childhood, polyarthralgias/polyarthritis, severe chronic red cell-aplasia in immunocompromised hosts, transient aplastic crises and miscarriage,4 although 20–30% of affected individuals do not develop symptoms.5 It has also been associated with other diseases, such as vasculitis, myocarditis, nephritis, immune thrombocytopenia, haemophagocytic lymphohistiocytosis and acute liver failure, but the causality of B19V in these diseases has not been confirmed.
The incubation period of B19V is generally of 1–2 weeks, but may last up to 21 days,3 and the illness follows a biphasic course. It starts with nonspecific flu-like symptoms associated with mild anaemia,2 while the second phase, due to the formation of immune complexes, is characterised by the development of the itchy rash and/or arthralgias,1 possibly accompanied by a second drop in the level of haemoglobin.2
Infected pregnant women may develop a rash or arthralgia, but approximately half remain asymptomatic.6 The rate of vertical transmission is of 17–33%,2 with transmission to the foetus occurring 1–3 weeks after the mother is infected.7 In most foetuses the disease resolves spontaneously, but there are 2 possible complications: hydrops fetalis and/or foetal death.2,7 The risk of foetal death ranges between 2% and 6%, and is higher when infection occurs in the second trimester of gestation.3 There are no known cases of associated congenital anomalies.6
Erythrovirus B19 is found worldwide and can cause sporadic infection or epidemic outbreaks, with a cyclical pattern of epidemic outbreaks occurring every 2–5 years.1,4 In 70% of cases, primary infection occurs during childhood or adolescence,1 and the prevalence of IgG antibodies against the virus in adults ranges from 40% to 60%, reaching 90% in the elderly.4
In 2015 we detected an increase in the detection of IgM antibodies against B19V, which led us to review the infections diagnosed in our hospital since serologic testing for B19V was first introduced in 2010 with the aim of determining the incidence in our catchment population. We also found that while B19V is broadly associated with childhood, there were many cases in adults, so we decided to review the clinical features in the adult and paediatric populations and the differences between them.
Materials and methodsWe reviewed data on the serologic tests for detection of B19V performed in our laboratory in the 2010–2015 period, and selected the cases with a diagnosis of acute infection by Erythrovirus B19. The presence of antigen-specific IgM is indicative or acute infection or recent exposure, as these antibodies appear 10–12 days after exposure and persists for 3–6 months.2 Specific IgG appears a few days following detection of IgM and persists for years at very low concentrations, indicating previous infection or immunity.3 We interpreted serologic results following the guidelines cited in the reference section: a positive IgM result with a negative IgG result is suggestive of acute infection and should be followed up with another test 1–2 weeks later to verify IgG seroconversion; positive results for both IgM and IgG suggest an acute or recent infection; a negative IgM result with a positive IgG result are indicative of past infection and immunity; and negative results for both IgM and IgG of a lack of exposure to or infection by the virus.2
Serologic tests were performed using the EVOLIS™ system (Bio-Rad Laboratories, Hercules, California, United States) and the LIAISON® Biotrin Parvovirus B19 IgG/IgM panel (DiaSorin, Dublin, Ireland) for detection of anti-Erythrovirus B19 IgG and IgM, with a positive result defined as an index greater than 1.1.
We conducted a retrospective study of the patients with a diagnosis of Erythrovirus B19 infection to establish the demographic, clinical and laboratory (haemoglobin levels, platelet count, white blood cell [WBC] count and serum level of C-reactive protein [CRP]) characteristics of this disease.
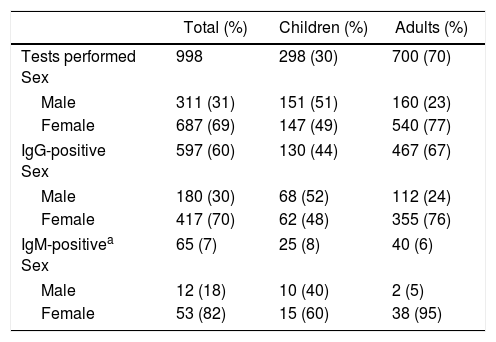
ResultsIn the 2010–2015 period, there were a total of 998 orders for antibody tests for Erythrovirus B19 (Table 1). The mean number of orders per year was 166, with a slight and statistically significant increase over time, from 151 in 2010 to 208 in 2015 (P<.01).
Serologic tests performed between 2010 and 2015.
| Total (%) | Children (%) | Adults (%) | |
|---|---|---|---|
| Tests performed Sex | 998 | 298 (30) | 700 (70) |
| Male | 311 (31) | 151 (51) | 160 (23) |
| Female | 687 (69) | 147 (49) | 540 (77) |
| IgG-positive Sex | 597 (60) | 130 (44) | 467 (67) |
| Male | 180 (30) | 68 (52) | 112 (24) |
| Female | 417 (70) | 62 (48) | 355 (76) |
| IgM-positivea Sex | 65 (7) | 25 (8) | 40 (6) |
| Male | 12 (18) | 10 (40) | 2 (5) |
| Female | 53 (82) | 15 (60) | 38 (95) |
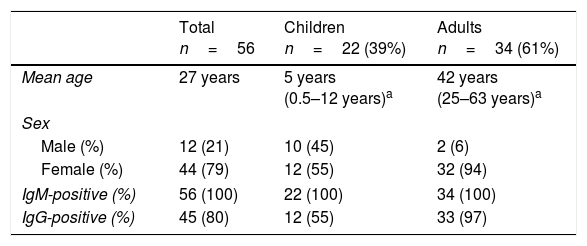
During this period, there were 56 diagnosed cases of acute infection Erythrovirus B19 (Table 2). We did not find significant differences in the distribution by sex in paediatric patients (P=.272), but there was a significant difference in incidence between the sexes in adults (P=.006).
Age, sex and serologic findings in the 56 patients with acute infection by Erythrovirus B19.
The incidence in this period was of 9.3cases/year, peaking in years 2012 and 2015, with 15 and 23 cases, respectively. Seventy-seven percent of cases occurred in spring or summer, with the annual incidence peaking between May and July, when 54% of the total cases clustered.
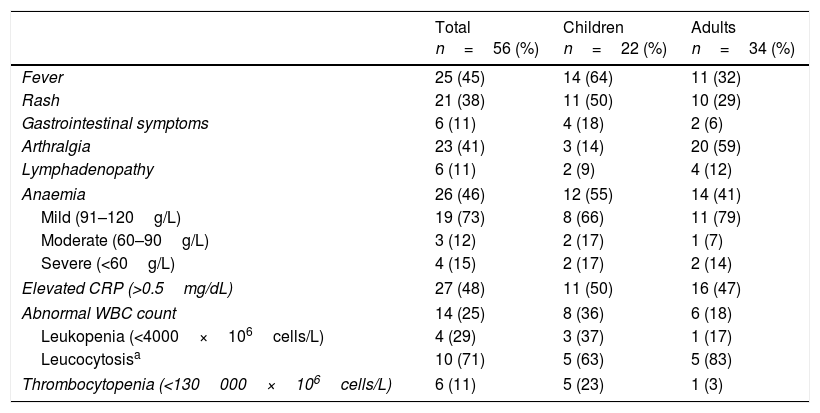
Table 3 presents the distribution of symptoms and laboratory findings.
Clinical and laboratory characteristics of the patients.
| Total n=56 (%) | Children n=22 (%) | Adults n=34 (%) | |
|---|---|---|---|
| Fever | 25 (45) | 14 (64) | 11 (32) |
| Rash | 21 (38) | 11 (50) | 10 (29) |
| Gastrointestinal symptoms | 6 (11) | 4 (18) | 2 (6) |
| Arthralgia | 23 (41) | 3 (14) | 20 (59) |
| Lymphadenopathy | 6 (11) | 2 (9) | 4 (12) |
| Anaemia | 26 (46) | 12 (55) | 14 (41) |
| Mild (91–120g/L) | 19 (73) | 8 (66) | 11 (79) |
| Moderate (60–90g/L) | 3 (12) | 2 (17) | 1 (7) |
| Severe (<60g/L) | 4 (15) | 2 (17) | 2 (14) |
| Elevated CRP (>0.5mg/dL) | 27 (48) | 11 (50) | 16 (47) |
| Abnormal WBC count | 14 (25) | 8 (36) | 6 (18) |
| Leukopenia (<4000×106cells/L) | 4 (29) | 3 (37) | 1 (17) |
| Leucocytosisa | 10 (71) | 5 (63) | 5 (83) |
| Thrombocytopenia (<130000×106cells/L) | 6 (11) | 5 (23) | 1 (3) |
We did not find any differences in presentation based on sex, but there were differences based on age, as fever and rash were more frequent in paediatric patients while arthralgia was the characteristic feature in adult patients.
Anaemia was frequent in both subsets of patients. We ought to note that of the 22 cases presenting with anaemia, the latter was mild to moderate in 22 cases, although it was severe in 4 with haemoglobin levels of less than 60g/L. These cases occurred in patients with underlying blood disorders: 2 children (homozygous sickle cell anaemia; history of haemolytic anaemia) and 2 adults (autoimmune haemolytic anaemia; systemic lupus erythematosus with chronic iron deficiency anaemia). Abnormal WBC counts were common in both groups, usually with an increased count. Thrombocytopenia was less frequent and usually mild, although it occurred in 23% of the paediatric patients, with platelet counts of less than 85±28.6×106/L.
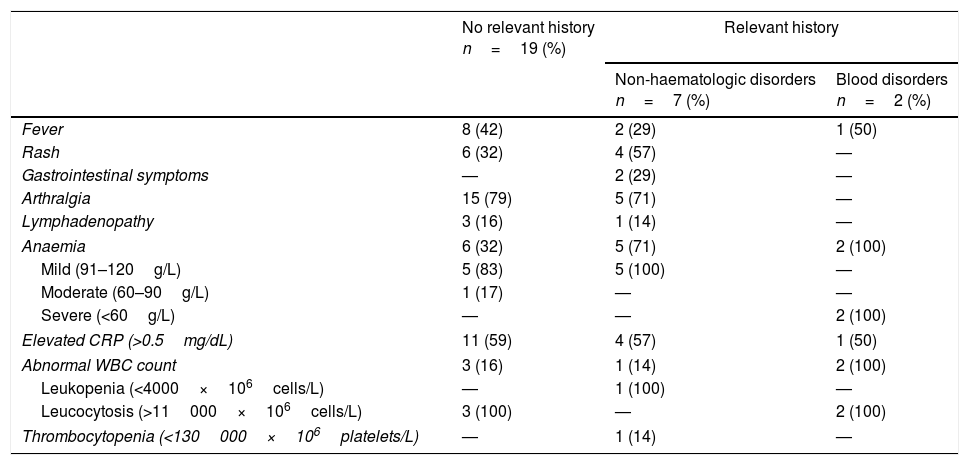
Of all paediatric patients, only 2 had a relevant medical history (the 2 patients with blood disorders). Table 4 summarises the data for the 28 cases in adult patients (excluding pregnant women) based on the presence (n=9) or absence (n=19) of pre-existing diseases. Two women in this group sought care due to contact with a direct relative exhibiting symptoms of infection by Erythrovirus B19. We did not find statistically significant differences in the presenting symptoms based on the medical history or presence of underlying disease except between patients with blood disorders, of who 100% presented with severe anaemia and 50% with fever, and patients with non-haematologic disorders, who presented with other signs and symptoms of B19V infection. All patients received symptomatic treatment with antipyretics and/or analgesics, except for patients with severe anaemia, who received transfusions of red blood cell concentrates. All had favourable outcomes.
Clinical and laboratory characteristics of adult patients with infection by Erythrovirus B19 (n=28) based on the presence or absence of a relevant clinical history, excluding pregnant women.
| No relevant history n=19 (%) | Relevant history | ||
|---|---|---|---|
| Non-haematologic disorders n=7 (%) | Blood disorders n=2 (%) | ||
| Fever | 8 (42) | 2 (29) | 1 (50) |
| Rash | 6 (32) | 4 (57) | — |
| Gastrointestinal symptoms | — | 2 (29) | — |
| Arthralgia | 15 (79) | 5 (71) | — |
| Lymphadenopathy | 3 (16) | 1 (14) | — |
| Anaemia | 6 (32) | 5 (71) | 2 (100) |
| Mild (91–120g/L) | 5 (83) | 5 (100) | — |
| Moderate (60–90g/L) | 1 (17) | — | — |
| Severe (<60g/L) | — | — | 2 (100) |
| Elevated CRP (>0.5mg/dL) | 11 (59) | 4 (57) | 1 (50) |
| Abnormal WBC count | 3 (16) | 1 (14) | 2 (100) |
| Leukopenia (<4000×106cells/L) | — | 1 (100) | — |
| Leucocytosis (>11000×106cells/L) | 3 (100) | — | 2 (100) |
| Thrombocytopenia (<130000×106platelets/L) | — | 1 (14) | — |
Documented non-haematologic disorders: Crohn disease; hypothyroidism and rheumatoid arthritis; atopic dermatitis; uterine fibroids; dyslipidaemia; nonspecific rheumatism and fibrositis; hypertension and diabetes mellitus.
Documented blood disorders: autoimmune haemolytic anaemia and systemic lupus erythematosus associated with chronic iron deficiency anaemia.
The other 6 cases detected in adults occurred in pregnant women. In 2 of them, testing was performed on account of their close contact with a child with infection by B19V, and in 1 as part of a workup following miscarriage, while the reason for ordering testing in the remaining 3 is unknown. In one case, the foetus had a completely normal development. In 3 cases, the foetus experienced abnormalities during gestation (polyhydramnios, oligoamnios and intrauterine growth restriction) without complications and with favourable outcomes after birth, but we could not establish whether these abnormalities were associated to the maternal infection by B19V. In the other 2 cases, the foetuses suffered severe complications of infection. In the first one, the mother sought care due to contact with her own child that had B19V infection, and the ultrasound scan at 26 weeks’ gestation revealed a massive foetal haemorrhage that required termination of the pregnancy. In the other case, the mother had a miscarriage due to foetal death at 11 weeks’ gestation, and the subsequent investigation revealed the presence of B19V-specific IgM antibodies. This was the only pregnant women in our series that experienced symptoms, a mild itchy rash in the lower extremities, 3 weeks before the miscarriage.
DiscussionWe found an incidence of infection by Erythrovirus B19 in our catchment population of 9.3cases/year. It is important to consider that 30% of infected individuals do not develop symptoms and that most that do have mild manifestations do not require medical attention or present with features (infectious rash) compatible with Erythrovirus B19 and thus do not require confirmation by serologic testing. For the above reasons, the actual incidence in the population is underestimated in our case series.
We did not find a different incidence in male versus female patients in the paediatric population, but we did find a significant difference based on sex in adult patients, with 82% of the cases diagnosed in women and a proportion of positive assay results of 7% in women 7% (38/540) compared to 1.2% in men (2/160). We hypothesis that the higher incidence in women could be explained by the higher frequency of arthralgia, especially of long duration, in women,3 which leads these patients to seek medical care, and by the more frequent contact with children, which may facilitate transmission of the virus.
As described in previous studies, we found that rash and fever were more common presentations in paediatric patients, while the most common presentation in adult patients was arthralgia, often associated with fever and/or rash. Anaemia was a frequent finding (46%) in both children and adults, but it was usually mild, with haemoglobin levels greater than 90g/L. However, in patients with underlying blood disorders the anaemia could be severe, triggering aplastic crises and requiring transfusions. Another frequent finding in both age groups was elevation of serum CRP levels and abnormal WBC counts with a tendency towards leucocytosis. We did not find any other significant differences in presentation based on the previous medical history.
We ought to emphasise the risk to the foetus during pregnancy. The incidence of infection by B19V in pregnant women is less than 4%,2 and most remain asymptomatic. The rate of vertical transmission is of less than 35%,7 but even though foetal infection can resolve spontaneously, there is also a risk of severe infection that may even be life-threatening, especially if it occurs before 20 weeks’ gestation. According to the American College of Obstetricians and Gynecologists (ACOG), once B19V infection is diagnosed, ultrasound scans should be performed weekly or every 15 days for 10–12 weeks to prevent hydrops fetalis. After 12 weeks, the risk of developing hydrops fetalis is nearly nonexistent.8
Serologic testing for Erythrovirus B19 (IgG and IgM) allows the diagnosis of acute infection. It is also possible to perform viral DNA testing in serum samples, but this method is more complex and expensive and offers no advantages over antibody assays except in cases of aplastic crisis and immunocompromised patients.3
The performance of antibody assays for Erythrovirus B19 in our laboratory allowed us to establish the characteristics of this infection in our region and identify specific clinical presentations of the associated illness that could otherwise not be given a correct aetiological diagnosis. We found while a total of 998 immunoassays were performed in our hospital, only 56 patients (5.6%) received a diagnosis of acute infection by B19V. However, it is important to take into account infection by B19V in cases of joint problems of recent onset and unknown aetiology, aplastic crisis in immunocompetent patients, red cell aplasia in immunocompromised patients and in pregnant women, as the identification of this aetiology is relevant in clinical decision making and can result in improved management of the patient.
In conclusion, while infection by Erythrovirus B19 can be a self-limiting disease with a mild course, especially in children, it can lead to severe complications requiring regular monitoring and/or treatment, especially in pregnant women, immunocompromised individuals or patients with underlying blood disorders.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Claver Belver N, Sanfeliu Sala I, Merino Asensio MJ, Monterde Pedra C, Pineda Solas V, Capilla Rubio S, et al. Infecciones por Erythrovirus B19. Seis años de seguimiento en población adulta y pediátrica. An Pediatr (Barc). 2019;90:280–284.
Previous presentations: This study was presented as a poster at the XXIV Jornadas de la Societat Catalana de Malalties Infeccioses i Microbiologia Clínica, October 23–24, 2015, St. Hilari Sacalm, Spain; and at the XX National Congress of the Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica, May 26–28, 2016, Barcelona, Spain.