Due to the emergence of new pharmaceutical presentations of ibuprofen (40mg/mL), an analysis was made on the use of antipyretics in paediatric outpatients in Spain.
Patients and methodsA cross-sectional, observational, descriptive study was carried out on a sample of children under 14 years old with treated febrile syndrome, seen in the Emergency Room of the Hospital General Universitario de Valencia from November 2012 to January 2013.
ResultsOf the 217 children included, 144 were treated with paracetamol or ibuprofen, 69 received both drugs, and one received paracetamol and metamizol. There were 58.7% of exposures to paracetamol and 40.9% to ibuprofen. The parents decided the use of antipyretics in 63.2% of cases. In 98 exposures the dose was different from that authorised in the labelling of the drug (off-label use). Ibuprofen was used off-label in 40.2% of cases, mostly by underdosing (35.9%). Paracetamol was used off-label in 29.8% of cases, predominantly overdose (26.8%), with the difference being statistically significant. No significant differences were observed in the off-label use in either monotherapy or combined use. There were also no differences when antipyretics prescribed by doctors or given directly by parents were evaluated separately.
ConclusionsThe majority of children with treated febrile syndrome seen in the Emergency Room were receiving antipyretic drugs after a parental decision. Paracetamol is the most commonly used drug and one in three children received it simultaneously with ibuprofen. The antipyretics were used off label in one-third of the cases. Off label use of ibuprofen is increasing, and is probably due to the existence of different pharmaceutical presentations.
Se analiza la utilización de antitérmicos en pediatría extrahospitalaria en España después de la aparición de medicamentos con 40mg/mL de ibuprofeno.
Pacientes y métodosEstudio transversal, observacional y descriptivo en pacientes menores de 14 años con síndrome febril ya tratado, atendidos en Urgencias del Servicio de Pediatría del Consorcio Hospital General Universitario de Valencia entre noviembre del 2012 y enero del 2013.
ResultadosDe 217 niños, 144 estaban tratados con paracetamol o ibuprofeno, 69 recibían ambos fármacos, y uno paracetamol y metamizol. Hubo un 58,7% de exposiciones a paracetamol y un 40,9% a ibuprofeno. En el 63,2% de los casos el uso de antitérmicos fue decidido por los padres. En 98 exposiciones la dosis fue diferente de la autorizada en la ficha técnica (uso «off-label»). El 40,2% de las exposiciones a ibuprofeno fue «off-label», en su mayoría por infradosificación (35,9%). Paracetamol se usó «off-label» en el 29,8% de los casos, predominando la sobredosificación (26,8%), diferencia estadísticamente significativa. No hubo diferencias significativas en el uso «off-label» combinado o en monoterapia.
ConclusionesLa mayoría de los niños con síndrome febril que llegan a urgencias con tratamiento antitérmico reciben este por decisión de sus padres. El paracetamol es el fármaco más usado, uno de cada 3 niños lo reciben simultáneamente con ibuprofeno. En un tercio de las ocasiones, los antitérmicos son utilizados al margen de la ficha técnica con una tendencia creciente que, en el caso del ibuprofeno, puede estar relacionada con la existencia de presentaciones farmacéuticas con distintas concentraciones.
Fever is a very frequent reason for paediatric visits. Although it is a physiological response of the body to various conditions, especially infection, it commonly generates anxiety and unwarranted fear in parents, a phenomenon that has been termed “fever phobia” that often leads to aggressive treatment of the fever.1,2
The drugs used most commonly to treat fever are paracetamol or ibuprofen, either alone or in alternation. The dosage forms used most frequently in paediatrics are ibuprofen oral suspensions and paracetamol solutions.1
Several studies1,3,4 show that the combined or alternating use of both drugs is not justified, because while it is more efficacious in reducing the temperature in the first hours of treatment, it carries a higher risk of dosage errors, and thus of incorrect use and potential liver and kidney toxicity. On the other hand, early or aggressive treatment of fever does not reduce the risk of febrile seizures, to the point that a recent update of British guidelines recommended using antipyretic drugs only when there is discomfort, and not with the sole purpose of bringing the body temperature down to normal.5
In Spain, different liquid formulations are available for both paracetamol and ibuprofen.6–8 Paracetamol is available in four different concentrations, but the one most commonly used is 100mg/mL. There are 10 brands, of which 7 correspond to this concentration. There are 2 ibuprofen liquid formulations. The oldest one, with the lower concentration, is the 20mg/mL formulation and it is either orange or white in colour. There are 13 different brands for this dosage form. In 2003 a more concentrated dosage form, with 40mg/mL, was introduced in the market, of which there are now 7 brands available. At first, the form with the lower concentration was used in younger children, and the one with the higher concentration in older children. In recent years, following the introduction in 2008 of a 40mg/mL formulation by the leading brand in the market, both concentrations started to be used interchangeably. Until January 2012, all the ibuprofen 40mg/mL formulations were pink or red, but at this time a new orange-coloured formulation was introduced. These circumstances can contribute to dosage errors.
The summary of product characteristics and the patient information leaflet of a drug, addressed to healthcare professionals and patients, respectively, contain the necessary information for the correct use of the medicine. In Spain, the use of drugs outside of what is authorised in the summary of product characteristics is referred to by the English term “off-label use,” as it is not covered by the information available in the label. There is no Spanish equivalent for this English expression, although perhaps the closest would be “uso al margen de la ficha técnica” (use outside of the summary of product characteristics). Neubert defined off-label use as the use of an approved drug in an unapproved way.9 This type of use must not be confused with the use of drugs under special circumstances, regulated by the Real Decreto (Royal Decree [RD]) 1015/2009, which refers to usage hitherto known as “compassionate”.
A study on the use of medication in outpatient paediatric patients found that in 30% of a total of 148 children with febrile syndrome, of which one third were being treated simultaneously with paracetamol and ibuprofen, there was off-label use of antipyretics.10,11
The aim of the present study, following the marketing of new formulations of ibuprofen, is to analyse the treatment of children with febrile syndrome seeking care at the paediatric emergency room having already received antipyretics, and to compare the actual use of antipyretics to the use authorised in the summaries of product characteristics.
Patients and methodsWe conducted a descriptive, observational, cross-sectional study on the use of antipyretic drugs during 3 consecutive months (November 2012–January 2013). We included all paediatric patients (children aged less than 14 years) who sought care in the paediatric emergency room of the Consorcio Hospital General Universitario de Valencia (CHGUV) with febrile syndrome that had been previously treated with antipyretic drugs. Each month, patients were recruited on 12 days chosen at random. We excluded patients with neoplastic disease, a known human immunodeficiency virus infection, or undergoing high-dose immunosuppresion therapy. Data were obtained by interviewing the parents or accompanying adults who were asked to consent to the data collection. We only included children whose caregivers agreed to participate. The study protocol was approved by the clinical research ethics committee of the CHGUV. We collected demographic and anthropometric data, as well as the following data for the antipyretic drugs: brand, active ingredient, dose, interval between doses, and whether it was a parent or a physician that decided to administer the drug. The dosage and intervals of administration used in each case were compared to the authorised ones as specified in their summaries of product characteristics (SPCs).6
The use of doses below 90% (underdose) or above 110% (overdose) of the dose authorised in the SPC (margin±10%) was considered off-label, as was the use of the drug in age groups other than those specified in the SPC. We reviewed the SPCs in the website of the Agencia Española de Medicamentos y Productos Sanitarios (Spanish Agency of Medicines and Health Products [AEMPS]): http://www.aemps.gob.es/cima/fichasTecnicas.do?metodo=detalleForm.
Statistical analysisWe collected the data in a form designed for that purpose. The statistical analysis was done using the Statistical Package for the Social Sciences (SPSS) version 15.0. We conducted a descriptive analysis. We have expressed the study results as the absolute and relative frequencies of the qualitative variables. We used the chi squared test to compare proportions for a 95% confidence interval.
ResultsWe collected data for 217 children. The mean age was 3.8 years (median, 2.9; maximum, 13.6; minimum, 0.33 years). All patients sought care for febrile syndrome, and were already taking antipyretic drugs. Of these children, 148 were taking a single antipyretic (99 paracetamol, 49 ibuprofen), while 69 were taking 2 (68 paracetamol and ibuprofen, and one paracetamol and metamizole). We analysed a total of 286 exposures to antipyretic drugs.
The most commonly used active ingredient was paracetamol (58.7% of exposures and 74.4% of children), followed by ibuprofen (40.9% of exposures and 53.9% of the children). The 20mg/mL preparation was used by 80.3% of the patients taking ibuprofen. We found a single prescription of metamizole in an alternating regime. The most frequent route of administration was the oral route (96.9%), and the rectal route was only used in 9 patients.
In most patients (63.9%) it was the parents that decided to administer the antipyretic drugs, while a physician prescribed them in approximately one third of patients (36.1%), a difference that was statistically significant (χ2=12.4). Parents chose to use paracetamol 61.7% of the time, and physicians 53.4% of the time. None of the patients were given antipyretic drugs following a recommendation by a pharmacist. Parents used antipyretic drugs as a single-agent therapy 53.0% of the times, and physicians 49.5% of the times.
We found the summary of product characteristics of all the drugs that appeared in our data, except for the summary for the paracetamol suppositories.
In 98 exposures (34.2%) the dose diverged from that authorised in the summary of product characteristics (off-label dosage), with similar percentages for underdosing and overdosing. We found no cases of off-label use based on age. Of the 98 off-label exposures, administration resulted from parental decision in 62, and was prescribed by paediatricians in 36. Table 1 summarises the key data on antipyretic usage.
Use of antipyretics by source of decision to treat (parent or physician).
| Used as authorised in SPC (%of total number of exposures) | Off-label (% of total number of exposures) | Overdose (% of off-label) | Underdose (% of off-label) | Total exposures | |
| Paediatricians | 67 (65.0) | 36 (35.0) | 17 (47.2) | 19 (52.8) | 103 |
| Parents | 121 (66.1) | 62 (33.9) | 32 (51.6) | 30 (42.4) | 183 |
| Total | 188 (65.7) | 98 (34.3) | 49 (50.0) | 49 (50.0) | 286 |
There is not statistically significant difference between the values for parents and paediatricians in any of the columns.
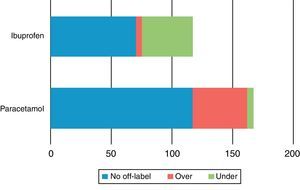
Administration of ibuprofen was off-label 40.2% of the time, most often due to underdosing (35.9%). Furthermore, 43.6% of exposures to the 20mg/mL formulation and 26.1% of exposures to the 40mg/mL formulation were off-label, a difference that was not statistically significant. The rate of off-label use was similar for the two formulations in medical prescriptions (41.7% for the 20mg/mL formulation and 45.4% for the 40mg/mL preparation), but when parents made the decision to treat, off-label use occurred more often for the lower concentration, in 50.0% of exposures versus 8.3% of exposures to the 40mg/mL formulation. The use of paracetamol was off-label in 29.8% of exposures, with a predominance of overdosing (26.8%), a statistically significant difference (χ2=20.9) (Fig. 1). The only case of exposure to metamizole was an overdose. Of the 49 instances of off-label use of paracetamol, the drug had been prescribed by a physician in 15 (30.6%). Ibuprofen was used off-label in 59 instances, having been prescribed by a physician in 21 (35.6%) of them.
Type of usage of the different antipyretics by number of patients. Metamizole was only used in one patient and was not represented (over=overdose, under=underdose). The difference in the frequencies of ibuprofen under- and overdosing between ibuprofen and paracetamol were statistically significant.
Off-label use occurred in 37.7% of the instances in which 2 antipyretics were used in combination. However, in patients treated with a single agent, off-label use amounted to 31.1% of exposures, a difference that was not statistically significant. Table 2 summarises the rate of off-label use in alternating and single-agent therapy when the decision to treat was made by parents and when it was made by paediatricians.
Off-label use by person who decided to treat and alternating or single-agent therapy.
| Alternating therapy (138 exposures) | Single-agent therapy (148 exposures) | |||||
| Total | Overdose | Underdose | Total | Overdose | Underdose | |
| Paediatricians | 17 (12.3%) | 10 (7.2%) | 7 (5.1%) | 19 (12.8%) | 7 (4.7%) | 12 (8.1%) |
| Parents | 35 (25.4%) | 13 (9.4%) | 22 (15.9%) | 27 (18.2%) | 19 (12.8%) | 8 (5.4%) |
| Total | 52 (37.7%) | 23 (16.7%) | 29 (21.0%) | 46 (31.1%) | 26 (17.6%) | 20 (13.5%) |
There is not statistically significant difference (P<.05) between the values for parents and paediatricians in any of the columns, or between alternating or single-agent therapy.
Each cell shows the absolute frequency, and the percentage of the total number of exposures in parenthesis.
Table 3 summarises the main results of this study, compared to those of a prior study.
Comparison of the off-label use of antipyretics in 2006 and in 2012–2013.
| Off-label use of antipyretics | |||
| 2006 | 2012–2013 | ||
| Total | 26.2% | 34.3% | Ns |
| Paracetamol | 31.3% | 29.8% | Ns |
| Ibuprofen | 20.2% | 40% | * |
| Parents | Total 40.4%Overdose 10.6%Underdose 29.8% | Total 33.9%Overdose 16.6%Underdose 18.4% | nsnsns |
| Paediatricians | Total 13.9%Overdose 4.6%Underdose 9.3% | Total 35%Overdose 18.6%Underdose 16.4% | **Ns |
| Alternating therapy | Total 3.9% | Total 38%Parents 40.7%(over 15.1%; under 25.6%)Paediatricians 32.7%(over 19.2%; under 13.5%) | * |
| Single-agent therapy | Total 52.6% | Total 31%Parents 27.8%(over 19.6%; under 8.2%)Paediatricians 37.3%(over 13.7%; under 23.6%) | Ns |
ns: not significant.
The results of this study show that the antipyretic agent used most commonly in paediatric patients with febrile syndrome in our setting is paracetamol, and that in approximately a third of the cases it is used in combination with ibuprofen. In most cases, antipyretic treatment is started by parents before visiting a healthcare facility. In 1 out of 3 cases, the dosage given was off the ranges specified in the summary of product characteristics, regardless of whether the decision to treat came from the parents or the paediatricians.
The frequency of combined antipyretic therapy was noteworthy, considering that this practise has not been proven to be more efficacious than single-agent therapy,3,4,12–14 and it has even been disadvised by guidelines for the use of antipyretics.1,5,15 Since we found no difference in the percentages of alternating therapy initiated by paediatricians versus parents, it is possible that the alternating use by paediatricians is precisely what leads parents to use 2 antipyretic agents when they make the decision to treat. A study conducted in Italy,16 where paediatric health services are organised in a similar manner as in Spain, showed that the decisions of paediatricians influence parental attitudes towards fever. However, the frequency of alternating therapy is lower in this study than that described by other authors,3 although perhaps this is affected by the data collection method. Since we conducted our study in an emergency room setting, the time since the onset of fever may have been shorter, making the initiation of an alternating regime less likely.
When we compared the data with those of the 2006 study,10,11 we observed an increasing trend in the use of paracetamol (from 53.4% to 58.7%) and a decreasing trend in the use of ibuprofen (from 46.6 to 40.9%). The percentage of the alternating use of antipyretics was similar in both studies (34% and 31%, respectively).
We found statistically significant differences in off-label use between paracetamol and ibuprofen, with a tendency to underdose ibuprofen, while overdosing occurred in 30% of paracetamol exposures. The risk involved in underdosing is therapeutic failure, but the use of doses above those recommended can result in toxicity.17
There are few studies on the off-label use of antipyretics. A study on analgesics reported that paracetamol accounted for 30% of off-label drug use due to incorrect dosage.18 Another study that collected information from databases showed that paracetamol was used off-label in 20.7% of cases, with underdosing being the most frequent reason for the off-label use.19 There is even less information for ibuprofen, but a recent study in a Portuguese paediatric emergency unit showed that it was used off-label in 18.9% of patients.20
The method commonly used by paediatricians to calculate dosages may account for the overdosing of paracetamol and the underdosing of ibuprofen. The recommended dose of paracetamol is about 15mg/kg/dose (maximum daily dose, 60mg/kg). The recommended ibuprofen dose is 5–10mg/kg/day. The most frequently used form of paracetamol is the 100mg/mL formulation, and the dose is usually calculated by multiplying the weight of the patient in kilograms by 0.15, which leads to its use at the maximum dose. Ibuprofen doses are usually calculating by dividing the patient's weight in kilograms by 3 for the 20mg/mL concentration and by 6 for the 40mg/mL concentration; for an administration of 6mg/kg/dose, at the low end of the therapeutic range.
When we compared the results of this study with those of the 2006 study, we observed that while the off-label use of paracetamol had not changed, the off-label use of ibuprofen had doubled in frequency, which may be due to the marketing of new dosage forms of the higher concentration. Off-label use in alternating therapy and in antipyretic use prescribed by paediatricians had also increased, especially when it came to overdosing.
Off-label use was distributed evenly between combined and single-agent therapy, regardless of whether it was the parent or paediatrician that chose to administer antipyretics. Underdosing was more frequent when antipyretic drugs were used in combination (59.1% in alternating therapy vs. 40.8% in single-agent therapy). This finding is heavily affected by the frequency of underdosing when parents alternate antipyretic agents (73.4%). As noted above, the parents’ behaviour usually reflects how paediatricians use antipyretics, except in the case of alternating therapy, in which parents underdose twice as frequently as paediatricians. This may be because parents have doubts when using more than one drug, and tend to underdose as a result.
The off-label use of antipyretic agents has grown in the past 6 years. This increase can be attributed to the fact that off-label use of these agents by paediatricians has increased considerably, by a factor of 2.5, with a higher frequency of overdose.
There are limitations to this study. The sample was small and the data collection period was short. However, we conducted it in the same setting as the previous study with which we are comparing it, so the characteristics of the patients may be similar. Another limitation may be the source of information, as it was obtained by means of interviews with parents, which may result in inaccuracies in the reported dosages used – these may be real, but they may not match the dosages prescribed by physicians. However, this method of data collection has been considered advantageous by some authors.21
The results of this study evince that there can be a disconnect between the available scientific evidence and clinical practise in the treatment of fever, which was also the conclusion of another study conducted in Switzerland.22 On the other hand, the fact that there are different concentrations available for a single active ingredient may contribute to the inappropriate use of antipyretics. In this regard, the Food and Drug Administration recently published a safety announcement about the availability of 2 concentrations of paracetamol and warning of the potential risks associated with it.23
To conclude, a third of the time antipyretics are used without adherence to the SPC. This is an increasing trend in the use of ibuprofen that may be due to the availability of dosage forms with different concentrations. As it is not possible that each antipyretic agent have a single dosage form, we should promote the training of paediatricians and parents in all aspects of fever and its correct treatment.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: García Blanes CP, Rodríguez-Cantón Pascual P, Morales-Carpi C, Morales-Olivas FJ. ¿Se ha modificado el uso de antitérmicos tras la introducción de ibuprofeno a diferentes concentraciones?. An Pediatr (Barc). 2014;81:383–388.