Late preterm infants (34–36 weeks gestation) have a morbidity rate significantly higher than those born at term. However, few interventions have been undertaken to reduce this increased morbidity and mortality. Antenatal corticosteroid administration could be an effective preventive measure.
ObjectiveThe aim of this study was to describe the morbidity associated with late prematurity in our institution, and determine if there are differences between those who received antenatal corticosteroids.
Patients and methodsA prospective observational study was conducted on late preterm infants born in a tertiary hospital from October 2011 until September 2012. Two groups were formed according to whether or not they had received antenatal steroids. The rates of morbidity and mortality for each of the groups were analysed and compared.
ResultsThere was a total of 4127 live newborns during the study period, of whom 3795 were term and 332 were preterm (the overall prematurity rate was 8.04%). There were 247 late preterm deliveries, representing 6% of live born infants, and 74.4% of all premature infants. Of late preterm infants, 63.2% were admitted to the Neonatal Unit and 29.6% had received antenatal steroids. The incidence of admission to the Neonatal Unit and Neonatal Intensive Care, transient tachypnea, need for respiratory support in the form of continuous positive pressure airway and oxygen therapy, incidence of hypoglycemia, feeding difficulty, and jaundice requiring phototherapy were significantly higher (P<0.05) in the late preterm group that did not receive antenatal steroids.
ConclusionsOur finding suggests that the administration of antenatal corticosteroids to patients at risk of 34–36 weeks delivery could significantly reduce the cost and acute morbidity associated with late preterm birth.
Se define al prematuro tardío como al recién nacido de 34 a 36 semanas de gestación. Este grupo presenta mayor riesgo de complicaciones que los nacidos a término. Sin embargo, son pocas las intervenciones que se realizan para reducir esta mayor morbimortalidad. La administración prenatal de corticoides podría ser una medida preventiva eficaz.
ObjetivoDescribir la morbilidad asociada a la prematuridad tardía y determinar si existen diferencias en los prematuros tardíos que recibieron corticoides prenatales.
Pacientes y métodosEstudio observacional prospectivo de los prematuros tardíos nacidos en un hospital terciario desde octubre de 2011 a octubre de 2012. Se clasificaron en 2grupos, según hubiesen o no recibido corticoides prenatales, y se compararon las tasas de morbimortalidad entre los 2grupos.
ResultadosLa tasa de prematuridad global fue del 8,04%, de los cuales el 74,4% (n=247) fueron prematuros tardíos. Precisaron ingreso el 63,2% (n=156), suponiendo el 17% del total de ingresados y el 20,6% de los ingresos en la unidad de cuidados intensivos neonatales. Recibieron corticoides prenatales el 29,6% (n=73). La incidencia de ingreso en neonatología y cuidados intensivos neonatales, la presencia de taquipnea transitoria, hipoglucemia, intolerancia digestiva, ictericia, asistencia respiratoria en forma de presión positiva continua en la vía respiratoria nasal, oxigenoterapia, sueroterapia y fototerapia fueron significativamente superiores (p<0,05) en el grupo que no recibió corticoides prenatales.
ConclusionesLa morbilidad de los prematuros tardíos de nuestro medio es significativamente inferior en los que recibieron corticoides prenatales, por lo que podría ser útil prolongar su administración más allá de las 34semanas.
Preterm birth is the primary cause of perinatal morbidity and mortality in developed countries. In the past few years, the rate of preterm births and the rate of survival of premature babies have both increased. Late preterm infants (LPIs), born between 34+0 and 36+6 weeks of gestational age, constitute the most frequent group (amounting to 75% of total premature births) and the one that has increased most significantly.1–3 Overall, they are at lesser risk than babies born at lower gestational ages, but recent studies have shown that due to their immaturity, their morbidity and mortality is greater than those of babies born at term.4–6 Although they are a high-risk group, few routine interventions have been established to reduce their higher rates of morbidity and mortality. One of the interventions that has most contributed to improve the prognosis of preterm NBs is the antenatal administration of corticosteroids.7 However, the foetal lung is considered to be mature at 34 weeks of gestation, at which point administration of corticosteroids is no longer indicated. As a result, few studies have evaluated the effect of antenatal corticosteroids in the late-preterm population.8–10
We set the following goals to assess the effect of antenatal corticosteroids in LPIs in our centre: to describe the morbidity associated to late prematurity and to determine if there are differences in the infants exposed to antenatal corticosteroids, as well as the associated prevalence, aetiology, and obstetric conditions.
Population and methodsWe conducted a prospective observational study the main purpose of which was assessing whether antenatal administration of corticosteroids in LPIs had an effect on their outcomes. We selected all patients born alive at 34+0 to 36+6 weeks of gestation in our hospital in a one-year period (October 1st, 2011 to September 30th, 2012). Gestational age was determined by obstetric criteria, based on the first day of the last menstrual period or on the ultrasound scan of the first half of the pregnancy. We calculated the overall rate of preterm birth and the rate of late preterm birth in the period under study.
We classified the selected NBs in 2 groups: (a) those that had been exposed to antenatal corticosteroids (prenatal administration of one or two 12-mg doses of betamethasone 24h apart) and (b) those that had not been exposed. We collected this information by reviewing medical records. The exclusion criteria were death before or during birth, presence of major congenital malformations, and presence of a confirmed or suspected genetic or metabolic disorder.
We analysed the following variables for the entire sample and for each of the groups: admission to the neonatal unit and/or the neonatal intensive care unit (NICU); length of hospital stay; mortality; relevant complications during pregnancy; mode of delivery; immediate resuscitation and perinatal period; respiratory disorders (hyaline membrane disease, transient tachypnoea of the newborn [TTNB], meconium aspiration syndrome, and pneumothorax) requiring respiratory support in the form of nasal continuous positive airway pressure (CPAP) or mechanical ventilation and administration of surfactant. We also analysed the incidence of jaundice requiring phototherapy, hypoglycaemia, digestive intolerance, need for fluid therapy and parenteral nutrition, and sepsis confirmed by clinical examination and blood culture.
Finally, we analysed all these parameters for each gestational age and birth weight group to determine whether there were any significant differences between infants that had been exposed to corticosteroids and infants that had not.
We compared the frequency of each of the parameters under study in each of the 2 groups. The statistical analysis was performed using SPSS version 18.0 for Windows. Quantitative variables are expressed as mean±standard deviation and maximums and minimums. Qualitative variables are expressed as absolute frequencies (n) and relative frequencies (%). We compared the groups using the chi squared test on contingency tables and Fisher's exact test when any of the expected frequencies was below 5.
ResultsIn the period under study (October 1, 2011 to September 30, 2012) there were 4127 NBs born alive, of whom 3795 were born at term and 332 born preterm, with an overall annual preterm birth rate of 8.04%. Among the latter group, 247 were late preterm, amounting to 6% of live births and 74.4% of all preterm births. Of all late preterm NBs, 63.2% were admitted to the neonatal unit (17% of all admitted NBs), for hospital stays lasting a mean of 14.70+11.4 (3–90) days. Among them, 28.2% required admission to the NICU for a mean length of hospital stay of 7.11±10.4 (1–70) days, amounting to 20.6% of the total number of NBs admitted to the NICU. None of the PTIs died during the neonatal period.
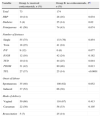
Table 1 describes the general characteristics, the obstetric and perinatal history, and the overall morbidity in the neonatal period of the PTIs included in this study.
General characteristics, obstetric and perinatal history, and overall morbidity of late preterm infants.
| Total | Absolute frequency (n=247) | Percentage (%) |
| General characteristics | ||
| Gestational age | ||
| 34 weeks | 49 | 19.8 |
| 35 weeks | 65 | 26.3 |
| 36 weeks | 133 | 53.8 |
| Birth weight | 2543(±496)g | |
| Male sex | 112 | 45.3 |
| Gestation | ||
| Maternal age ≥35years | 105 | 42.5 |
| HBP | 38 | 15.4 |
| Diabetes | 28 | 11.3 |
| Primiparous | 115 | 46.6 |
| Twin gestation | 59 | 23.9 |
| IVF | 19 | 7.7 |
| IUGR | 54 | 21.9 |
| TPL | 52 | 21.1 |
| PROM | 111 | 44.9 |
| Delivery | ||
| Onset | ||
| Spontaneous | 144 | 58.3 |
| Induced | 103 | 41.7 |
| Mode | ||
| Vaginal | 165 | 67.6 |
| Caesarean | 82 | 32.4 |
| Requiring resuscitation | 30 | 12.1 |
| Overall morbidity | ||
| TTNB | 50 | 20.2 |
| HMD | 13 | 5.3 |
| Pneumothorax | 1 | 0.4 |
| PHT | 3 | 1.2 |
| Oxygen therapy | 16 | 6.5 |
| CPAP | 46 | 18.6 |
| CMV | 17 | 6.9 |
| Surfactant therapy | 14 | 5.7 |
| Hypoglycaemia | 19 | 7.7 |
| Digestive intolerance | 106 | 42.9 |
| Fluid therapy | 95 | 38.5 |
| NEC | 1 | 0.4 |
| Parenteral nutrition | 36 | 14.6 |
| Phototherapy | 125 | 50.6 |
| Confirmed sepsis | 8 | 3.2 |
CMV: conventional mechanical ventilation; CPAP: continuous positive airway pressure; HBP: high blood pressure; HMD: hyaline membrane disease; IVF: in vitro fertilisation; IUGR: intrauterine growth restriction; NEC: necrotising enterocolitis; PHT: pulmonary hypertension; PROM: premature rupture of membranes; TPL: threatened preterm labour; TTNB: transient tachypnoea of the newborn.
The PTIs in the study (n=247) were divided into 2 groups according to the antenatal administration of corticosteroids. Antenatal corticosteroids were administered in 29.6% (n=73), and 98.6% (n=72) of them received a complete course (2 doses of betamethasone 24h apart before delivery). The mean gestational age at administration was 31+6±2+2 (25–34+1) weeks. The mean time elapsed from administration to delivery was 27.2±15.4 (0–65) days. In the 12.5% women that received a full course, delivery occurred within 7 days from its administration.
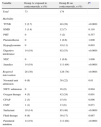
We found no significant differences between the groups in most of their perinatal histories and outcomes (Table 2).
Comparative study of perinatal history and outcomes according to administration of antenatal corticosteroids in late preterm infants.
| Variable | Group A: received corticosteroids, n (%) | Group B: no corticosteroids, n (%) | Pa |
| Total | 73 | 174 | |
| HBP | 10 (14) | 28 (16) | 0.634 |
| Diabetes | 3 (4) | 25 (14) | 0.02 |
| Primiparous | 41 (56) | 74 (43) | 0.074 |
| Number of foetuses | |||
| Single | 55 (75) | 133 (76) | 0.854 |
| Twin | 18 (25) | 41 (24) | |
| IVF | 9 (12) | 0 (6) | 0.077 |
| IUGR | 12 (16) | 42 (24) | 0.182 |
| TFD | 10 (14) | 44 (25) | 0.044 |
| PROM | 31 (42) | 80 (46) | 0.613 |
| TPL | 27 (37) | 25 (14) | <0.0001 |
| Onset of labour | |||
| Spontaneous | 35 (48) | 108 (62) | 0.052 |
| Induced | 37 (52) | 66 (38) | |
| Mode of delivery | |||
| Vaginal | 50 (68) | 116 (67) | 0.413 |
| Caesarean | 22 (30) | 58 (33) | 0.105 |
| Resuscitation | 5 (7) | 25 (14) | |
HBP: high blood pressure; IUGR: intrauterine growth restriction; IVF: in vitro fertilisation; PROM: premature rupture of membranes; TFD: threatened foetal distress; TPL: threatened preterm labour.
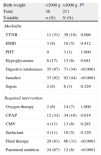
The results of the comparative study of both groups for neonatal morbidity and required interventions are shown in Table 3.
Comparative study of morbidity and mortality and required interventions in late preterm infants by antenatal corticosteroid administration.
| Variable | Group A: exposed to corticosteroids, n (%) | Group B: no corticosteroids, n (%) | Pa |
| Total | 73 | 174 | |
| Morbidity | |||
| TTNB | 2 (2.7) | 48 (28) | <0.0001 |
| HMD | 1 (1.4) | 12 (7) | 0.116 |
| PHT | 0 | 3 (2) | 0.557 |
| Pneumothorax | 0 | 1 (0.6) | 1.000 |
| Hypoglycaemia | 0 | 19 (11) | 0.003 |
| Digestive intolerance | 14 (19) | 92 (53) | <0.0001 |
| NEC | 0 | 1 (0.6) | 1.000 |
| Jaundice | 14 (19) | 111 (64) | <0.0001 |
| Required intervention | 28 (38) | 128 (74) | <0.0001 |
| Neonatal unit admission | 6 (8) | 38 (22) | 0.01 |
| NICU admission | 0 | 16 (9) | 0.004 |
| Oxygen therapy | 4 (5) | 42 (24) | 0.001 |
| CPAP | 2 (3) | 15 (9) | 0.096 |
| CMV | 1 (1) | 13 (8) | 0.071 |
| Surfactant | 10 (14) | 85 (49) | <0.0001 |
| Fluid therapy | 6 (8) | 30 (17) | 0.067 |
| Parenteral nutrition | 14 (19) | 111 (64) | <0.0001 |
CMV: conventional mechanical ventilation; CPAP: continuous positive airway pressure; HMD: hyaline membrane disease; NEC: necrotising enterocolitis; NICU: neonatal intensive care unit; PHT: pulmonary hypertension; TTNB: transient tachypnoea of the newborn.
The rate of admission to the neonatal unit was significantly higher (74% vs 38%) in LPIs that had not been exposed to antenatal corticosteroids. The rate of NICU admission was also significantly higher in this group (22% vs 8%). All the diseases associated to late preterm birth were less frequent in the group exposed to antenatal corticosteroids, and the differences were significant for TTNB, the presence of hypoglycaemia, digestive intolerance, and jaundice requiring phototherapy. The need for respiratory support by means of oxygen therapy and nasal CPAP and the need for fluid therapy and parenteral nutrition were also significantly higher in the groups of LPI that were not exposed to antenatal corticosteroids.
The comparative analysis by gestational age showed significant differences on a week to week basis, with a clear decrease of morbidity as gestational age increased (Table 4). All the pathologies and needs for interventions were less frequent in neonates who had been exposed to antenatal corticosteroids at any gestational age, and once again the decrease was significant for TTNB, digestive intolerance, jaundice requiring phototherapy, fluid therapy, and nasal CPAP (Table 5).
Comparative study of morbidity and required interventions by gestational age.
| Age | 34–34+6 weeks | 35–35+6 weeks | 36–36+6 weeks | |
| Total | 49 | 65 | 133 | |
| Variable | n (%) | n (%) | n (%) | Pa |
| Morbidity | ||||
| TTNB | 20 (41) | 7 (26) | 13 (10) | <0.0001 |
| HMD | 10 (20) | 2 (3) | 1 (0.8) | <0.0001 |
| PHT | 1 (2) | 1 (1.5) | 1 (0.8) | 0.751 |
| Hypoglycaemia | 5 (10) | 5 (8) | 9 (7) | 0.742 |
| Digestive intolerance | 44 (90) | 35 (64) | 27 (21) | <0.0001 |
| Jaundice | 44 (90) | 46 (71) | 35 (26) | <0.0001 |
| Sepsis | 3 (6) | 3 (5) | 2 (2) | 0.226 |
| Required intervention | ||||
| Oxygen therapy | 8 (16) | 6 (9) | 2 (1.5) | 0.001 |
| CPAP | 25 (51) | 14 (22) | 7 (5) | <0.0001 |
| CMV | 11 (22) | 3 (5) | 3 (2) | <0.0001 |
| Surfactant | 11 (22) | 2 (3) | 1 (0.8) | <0.0001 |
| Fluid therapy | 35 (71) | 34 (62) | 26 (20) | <0.0001 |
| Parenteral nutrition | 20 (41) | 8 (12) | 8 (6) | <0.0001 |
CMV: conventional mechanical ventilation; CPAP: continuous positive airway pressure; HMD: hyaline membrane disease; PHT: pulmonary hypertension; TTNB: transient tachypnoea of the newborn.
Comparative study of morbidity and required interventions by gestational age and administration of antenatal corticosteroids.
| Variable | TTNB, n (%) | HMD, n (%) | Hypoglycaemia, n (%) | Digestive intolerance, n (%) | Jaundice, n (%) | Sepsis, n (%) |
| 34–34+6weeks (n=49) | ||||||
| CS (n=14) | 1 (7) | 1 (7) | 0 | 10 (71) | 10 (71) | 0 |
| No CS (n=35) | 19 (54) | 9 (26) | 5 (14) | 34 (97) | 34 (97) | 3 (9) |
| Pa | 0.002 | 0.244 | 0.303 | 0.019 | 0.019 | 0.548 |
| 35–35+6weeks (n=65) | ||||||
| CS (n=17) | 1 (6) | 0 | 0 | 3 (18) | 4 (23) | 1 (6) |
| No CS (n=48) | 16 (33) | 2 (4) | 5 (10) | 32 (67) | 42 (88) | 2 (4) |
| Pa | 0.029 | 1.000 | 0.315 | <0.0001 | <0.0001 | 1.000 |
| 36–36+6weeks (n=133) | ||||||
| CS (n=42) | 0 | 1 (1) | 0 | 1 (2) | 0 | 0 |
| No CS (n=91) | 13 (14) | 1 (1) | 9 (10) | 26 (29) | 35 (39) | 2 (2) |
| Pa | 0.009 | 1.000 | 0.057 | <0.0001 | <0.0001 | 1.000 |
| Variable | Oxygen, n (%) | CPAP, n (%) | CMV, n (%) | Surfactant, n (%) | Fluid therapy, n (%) | Parenteral nutrition, n (%) |
| 34–34+6weeks (n=49) | ||||||
| CS (n=14) | 0 | 2 (14) | 1 (7) | 1 (7) | 7 (50) | 5 (36) |
| No CS (n=35) | 8 (23) | 23 (66) | 10 (29) | 10 (29) | 28 (80) | 15 (43) |
| Pa | 0.085 | 0.001 | 0.143 | 0.143 | 0.08 | 0.646 |
| 35–35+6weeks (n=65) | ||||||
| CS (n=17) | 0 | 1 (6) | 0 | 0 | 2 (12) | 1 (6) |
| No CS (n=48) | 6 (13) | 13 (27) | 2 (4) | 2 (4) | 32 (67) | 7 (15) |
| Pa | 0.327 | 0.009 | 1.000 | 1.000 | <0.0001 | 0.669 |
| 36–36+6weeks (n=133) | ||||||
| CS (n=42) | 0 | 1 (2) | 0 | 0 | 2 (12) | 0 |
| No CS (n=91) | 2 (2) | 6 (7) | 1 (1) | 1 (1) | 32 (67) | 8 (9) |
| Pa | 1.000 | 0.43 | 1.000 | 1.000 | <0.0001 | 0.056 |
CMV: conventional mechanical ventilation; CPAP: continuous positive airway pressure; CS: corticosteroids; HMD: hyaline membrane disease; TTNB: transient tachypnoea of the newborn.
Lastly, when we analysed the data by birth weight we confirmed that there is increased risk at lower birth weights, especially of feeding difficulties and the need for fluid therapy and parenteral nutrition, which were significantly more frequent (Table 6). Morbidity was higher in all LPIs that were not exposed to antenatal corticosteroids, although the differences were only significant for infants with birth weights below 2000g, which constituted the largest group (Table 7).
Comparative study of morbidity and required interventions by birth weight.
| Birth weight | <2000g | >2000g | Pa |
| Total | 36 | 211 | |
| Variable | n (%) | N (%) | |
| Morbidity | |||
| TTNB | 11 (31) | 39 (18) | 0.096 |
| HMD | 3 (8) | 10 (5) | 0.412 |
| PHT | 0 | 3 (1) | 1.000 |
| Hypoglycaemia | 6 (17) | 13 (6) | 0.041 |
| Digestive intolerance | 35 (97) | 71 (34) | <0.0001 |
| Jaundice | 33 (92) | 92 (44) | <0.0001 |
| Sepsis | 2 (6) | 6 (3) | 0.329 |
| Required intervention | |||
| Oxygen therapy | 2 (6) | 14 (7) | 1.000 |
| CPAP | 12 (33) | 34 (16) | 0.014 |
| CMV | 4 (11) | 13 (6) | 0.285 |
| Surfactant | 4 (11) | 10 (5) | 0.129 |
| Fluid therapy | 29 (81) | 66 (31) | <0.0001 |
| Parenteral nutrition | 24 (67) | 12 (6) | <0.0001 |
VMC: conventional mechanical ventilation; CPAP: continuous positive airway pressure; HMD: hyaline membrane disease; PHT: pulmonary hypertension; TTNB: transient tachypnoea of the newborn.
Comparative study of morbidity and mortality and required interventions by birth weight and administration of antenatal corticosteroids.
| Variable | TTNB, n (%) | HMD, n (%) | Hypoglycaemia, n (%) | Digestive intolerance, n (%) | Jaundice, n (%) | Sepsis, n (%) |
| <2000g (n=36) | ||||||
| CS (n=7) | 1 (14) | 1 (14) | 0 | 7 (100) | 6 (86) | 0 |
| No CS (n=29) | 10 (34) | 2 (7) | 6 (21) | 28 (97) | 27 (93) | 2 (7) |
| Pa | 0.400 | 0.488 | 0.317 | 1.000 | 0.488 | 1.000 |
| >2000g (n=211) | ||||||
| CS (n=66) | 1 (2) | 0 | 0 | 7 (11) | 8 (12) | 1 (2) |
| No CS (n=145) | 38 (26) | 10 (7) | 13 (9) | 64 (44) | 84 (58) | 5 (3) |
| Pa | <0.0001 | 0.033 | 0.011 | <0.0001 | <0.0001 | 0.668 |
| Variable | Oxygen, n (%) | CPAP, n (%) | CMV, n (%) | Surfactant, n (%) | Fluid therapy, n (%) | Parenteral nutrition, n (%) |
| <2000g (n=36) | ||||||
| CS (n=7) | 0 | 2 (29) | 1 (14) | 1 (14) | 3 (43) | 6 (86) |
| No CS (n=29) | 2 (7) | 10 (34) | 3 (10) | 3 (10) | 26 (90) | 18 (62) |
| Pa | 1.000 | 1.000 | 1.000 | 1.000 | 0.016 | 0.384 |
| >2000g (n=211) | ||||||
| CS (n=66) | 0 | 2 (3) | 1 (2) | 0 | 7 (11) | 0 |
| No CS (n=145) | 14 (10) | 32 (22) | 12 (8) | 10 (7) | 59 (41) | 12 (8) |
| Pa | 0.006 | <0.0001 | 0.068 | 0.033 | <0.0001 | 0.020 |
CMV: conventional mechanical ventilation; CPAP: continuous positive airway pressure; CS: corticosteroids; HMD: hyaline membrane disease; TTNB: transient tachypnoea of the newborn.
Consistent with the reviewed literature, the overall rate of preterm birth in our hospital during the period under study was 8.04%. Within this group, 74.4% of patients belonged to the LP subgroup. These data are in agreement with those published in most developed countries, where increasing trends have also been reported.1,11,12 Some of the underlying causes are the older age of primiparous mothers, multiple pregnancies, assisted reproductive technology, and an increase in the indications for induced labour and scheduled caesarean delivery. In this study, 42.5% of the mothers were 35 or more years old; 46.6% were primiparous mothers; 7.7% of pregnancies were the result of assisted reproduction; 24.1% of pregnancies were multiple gestations; and 41.7% of deliveries were induced due to maternal or foetal indications, figures that are consistent with those reported in the literature.13–17 These results make sense considering that all these factors are interrelated. Advanced maternal age is associated with a higher risk of obstetric complications, which are often indications for the early termination of pregnancy, and is more frequently associated with the need for assisted reproduction technologies, which increase the probability of a multiple pregnancy.
In our study, 63.2% of LPIs were admitted to the neonatal unit, and 28.2% required intensive care, giving rise to a considerable number of hospital admissions, as this group is the largest among preterm infants. They account for 17% of the total NB admissions, and for 20.6% of NICU admissions. The general criteria for admission in our hospital are having a gestational age of 34 weeks or less; or having a gestational age of more than 34 weeks and a birth weight below 2300g or suffering from any disease that requires monitoring and/or treatment.
In the past decade, numerous studies have confirmed that these infants, due to their immaturity, have higher morbidity than full-term infants. The LPIs in our study had rates of respiratory disorders, jaundice, hypoglycaemia, feeding difficulties, and sepsis similar to those reported in the literature.4,18–22
The evidence shows that pharmacological induction of foetal lung maturity by means of corticosteroids is the intervention that most improves the prognosis of preterm NBs. Consequently, Spanish and American associations of obstetricians and gynaecologists recommend antenatal administration of corticosteroids to pregnant women at risk of preterm delivery between 24 and 34 weeks of gestation in order to reduce the incidence of acute respiratory distress syndrome, intraventricular haemorrhage, necrotising enterocolitis, and/or neonatal death.23
In our study, 29.6% (73) of LPIs had received antenatal corticosteroids, and admission rates were significantly higher in those who had not received them, both for the neonatal unit (74% vs 38%) and the NICU (22% vs 8%).
Respiratory disorders are among the most frequent morbidities associated to late preterm birth. In this study, 27.1% of LPIs developed respiratory complications, with TTNB being the most frequent diagnosis (20.2%). This high incidence could be due to abnormalities or delays in the clearance of foetal lung fluid after birth. Various studies propose that sodium transport across the alveolar epithelium is the main mechanism involved in foetal lung fluid clearance, one which develops as gestational age increases and that becomes particularly important in the last weeks of pregnancy. In LPIs, the lower number as well as the immaturity of sodium transport mechanisms may be involved in the pathogenesis of TTNB.24 The incidence of TTNB in the no-steroids group of LPIs was significantly higher than in the steroid-exposed group: 28% versus 2.7% (P<0.0001). This difference may be explained by the fact that only corticosteroids seem to stimulate the synthesis and activity of sodium channels in the alveolar epithelium, contributing to the reabsorption of lung fluid. There is evidence that a surge of endogenous steroids and catecholamines accompanies term gestation and spontaneous vaginal delivery, and that these hormonal changes are necessary for foetal maturation and to prepare the foetus to transition successfully to neonatal life. This would account for the higher incidence of TTNB in LPIs who have not been exposed to corticosteroids, especially in those delivered by caesarean section, a procedure that is more common in the latter group (32.4%).25 In our study, the same proportion of infants with TTNB had been born vaginally and by caesarean delivery. In each group, only one neonate had been exposed to antenatal corticosteroids. Our results suggest that antenatal corticosteroids reduce the risk of TTNB, regardless of the type of delivery.
This issue is important because TTNB is a frequent complication and the main reason for hospital admission in LPIs. The rate of morbidity should not be the only factor in deciding which is the gestational age at which administration of antenatal corticosteroids should be recommended, (LPIs have a lower rate of respiratory morbidity than preterm infants with lower gestational ages); the total number of patients that could benefit also ought to be considered. As noted above, LPIs constitute the largest group of preterm infants, and antenatal treatment could reduce the number of patients admitted to the NICU, resulting in a lower socioeconomic burden.26–28 Likewise, the Cochrane review supports antenatal administration of corticosteroids up to 346+ weeks of gestation. This recommendation is based on the reduction in the incidence of respiratory distress syndrome in the subgroup of preterm infants born at 33–34+6 weeks of gestation exposed to antenatal corticosteroids.29
In this study, the incidence of hyaline membrane disease was very low in both groups, and we found no significant differences were between them. However, both this condition and surfactant administration were more frequent in LPIs who were not exposed to antenatal corticosteroids, which could be due to the structural and functional development of the foetal lung, which is incomplete in this gestational age group.
There are very few studies on the probable beneficial effect of antenatal corticosteroid administration in late preterm infants. To obtain evidence on this matter, the Maternal Fetal Medicine Units Network is conducting a prospective multicentre study (Antenatal Late Preterm: Randomized Placebo-Controlled Trial [ALPS]) to evaluate the administration of antenatal corticosteroids in women at risk of preterm delivery between 34 and 36+6 weeks of gestation, which will be completed in 2014.26
Respiratory morbidity is not only the primary cause for admission in LPIs, but is accompanied by additional complications such as digestive intolerance, which may or may not be associated with the need for respiratory support measures, fluid therapy, and parenteral nutrition. These findings are corroborated by our study, as our results showed a significant reduction not only in the risk of acute respiratory diseases (TTNB), but also in the risk of developing hypoglycaemia, digestive intolerance and jaundice, and of needing respiratory support (oxygen therapy, nasal CPAP), fluid therapy and phototherapy among the LPIs exposed to antenatal corticosteroids. Thus, based on our results, the extension of antenatal corticosteroid administration to LPIs would be an effective strategy to reduce the length of hospital stays and the consumption of resources.
The 34-week cutoff is still an arbitrary one. While the lung is traditionally considered to have reached structural and biochemical maturity by this gestational age, our data are not consistent with this. When we analysed morbidity by gestational age, we found a significant reduction in risk as gestational age increased; however, the beneficial effect of antenatal corticosteroid treatment was observed throughout the late preterm period, a finding that was independent of birth weight. Furthermore, corticosteroids not only affect the structural and functional maturity of the foetal lung, but also have extrapulmonary effects that contribute to the maturation of different organs and systems.7,30,31
There are studies that do not support the antenatal administration of corticosteroids. The observational multicentre study by Gyamfi-Bannerman et al.32 did not show an association between antenatal corticoid exposure and a reduction of respiratory morbidity in LPIs. A significant limitation of this study was that none of the mothers were given corticosteroids between 34 and 36 weeks of gestation, and that the time of administration was unknown, a factor to consider given that the effect of corticosteroids decreases with the passage of time. In our study, most mothers received a full course of corticosteroids before 34 weeks of gestation (the mean gestational age at administration was 31+6±2+2 weeks). The time elapsed to delivery was 27.2±15.4 (0–65) days, and birth occurred within 7 days of administration in 12.5% of the neonates.
Another study is a clinical trial in Brazil with a sample of 320 women between 34 and 36 weeks of gestation who were given a course of corticosteroids or placebo. The rate of acute respiratory distress syndrome was very low in both groups, and the rate of TTNB was similar and high in both groups, so the study did not reach sufficient power to detect significant differences.33
Up to now, no clinical trial has evaluated the long-term effects of the administration of antenatal corticosteroids in the late preterm period. The effects described most frequently are growth and neurodevelopmental disorders. Animal models show that corticosteroids induce apoptosis and cell death in the brains of exposed animals. In most humans, the process of neuronal cell division is already complete by 24 weeks of gestation, but the division of oligodendrocytes, the main cells involved in myelin synthesis, is not complete, as they grow most rapidly between 34 and 36 weeks of gestation. This would make LPIs more vulnerable to the potential adverse neurologic effects of antenatal corticoids than more preterm infants.34 A recent study in Sweden suggests that the beneficial effects of antenatal corticosteroids extends beyond 34 weeks and does not seem to increase the risk of adverse neurologic effects.35 All of the above justifies the need to make a long-term follow up of this large group of preterm infants, and to conduct more studies on the potential adverse effects of corticosteroids before making a definitive recommendation.
One of the most feared short-term adverse effects is the potential to increase the risk of perinatal infection in LPIs, a risk that increases due to the immaturity of their immune systems.36 This led us to assess and compare the incidence of sepsis, and we found no difference between groups. Thus, in our study, the use of antenatal corticosteroids did not increase the risk of infection in LPIs.
ConclusionIn our setting, the morbidity of LPIs, and respiratory morbidity in particular, is significantly lower in those that had been exposed to antenatal corticosteroids, who did not experience adverse effects in the short-term. If an absence of adverse effects in the long-term is confirmed, it may be useful to prolong the administration of antenatal corticosteroids beyond 34 weeks of gestation, with the purpose of significantly reducing morbidity and mortality rates, the length of hospital stays, the need for admission to the NICU, the use of resources, and the socio-economic burden associated with this subset of the population.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Gázquez Serrano IM, Arroyos Plana A, Díaz Morales O, Herráiz Perea C, Holgueras Bragado A. Corticoterapia prenatal y morbimortalidad del prematuro tardío: estudio prospectivo. An Pediatr (Barc). 2014;81:374–382