The population of late preterm infants (PT), those born between 34+0 and 36+6 weeks of gestation, accounts for 70–74% of all premature infants, and is not specifically included in most of the follow-up protocols for preterm infants. For many years, PTs have been handled as if they were term newborns, which has led to a limited knowledge of their outcome in the medium and long term. Their neonatal morbidity is associated with a higher incidence of postnatal complications, with an increased rate of hospital re-admissions due to malnutrition, hyperbilirubinaemia, and respiratory problems, when compared to term infants. Cerebral immaturity may be the main cause of the deficits observed in the long-term neurodevelopment of this population, making them more vulnerable. Several issues have been described, such as delays or disabilities in the pre-school stage, cerebral palsy, mental retardation, intellectual disability, schizophrenia, and psychological development of behavioural and emotional disorders.
The SEN34-36 Group of the Spanish Society of Neonatology, in collaboration with the Spanish Association of Primary Care Paediatrics, have developed these follow-up recommendations with the main objective of reducing the impact of prematurity on PT development. The secondary objectives of the document are to make neonatologists and paediatricians aware of the risks of sequelae of PTs, to determine and unify the evaluations and/or interventions that should be carried out, to offer clinical follow-up tools for the early detection of developmental delays, and to coordinate the care by all the professionals involved.
La población de prematuros tardíos (PT), aquellos nacidos entre las 340 y 366 semanas de gestación, representa el 70-74% de todos los prematuros, y no está incluida de forma específica en la mayoría de los protocolos de seguimiento para niños prematuros. Durante muchos años los PT han sido manejados como si de recién nacidos a término se tratasen, lo que ha llevado al desconocimiento de su evolución a medio y largo plazo. A la morbilidad neonatal se añade una mayor incidencia de afección posnatal, con una tasa de reingresos hospitalarios por malnutrición, hiperbilirrubinemia y problemas respiratorios superior a los nacidos a término. La inmadurez cerebral puede ser el principal responsable de los déficits observados en el neurodesarrollo a largo plazo en esta población y aumentar su vulnerabilidad. Se describen retrasos o discapacidades en la etapa preescolar, parálisis cerebral, retraso mental, discapacidad intelectual, esquizofrenia, trastornos del desarrollo psicológico, la conducta y la emoción.
El grupo SEN34-36 de la Sociedad Española de Neonatología, en colaboración con la Asociación Española de Pediatría de Atención Primaria, han desarrollado estas recomendaciones de seguimiento con el objetivo principal de disminuir el impacto de la prematuridad en el desarrollo de los PT. Los objetivos secundarios del documento son sensibilizar a neonatólogos y pediatras de los posibles riesgos de secuelas de los PT, determinar y unificar las evaluaciones y/o intervenciones que deberían realizarse, ofrecer herramientas de seguimiento clínico para detectar de manera precoz los déficits en el desarrollo y coordinar la atención de todos los profesionales implicados.
Preterm birth continues to be the main cause of morbidity and mortality in infants and children, and is one of the most important health problems in society, especially in developed countries. Late preterm (LPT) infants, born between 34+0 and 36+6 weeks’ gestation, amount to 70–74% of all preterm infants, and have not been included in most of the protocols and/or guidelines for the follow-up of children born preterm. This is because children born preterm have been managed as if they had been born at term for many years, resulting in a lack of knowledge on their long-term outcomes. However, this subset of preterm infants has been the object of many studies in the past decade, resulting in evidence of differences in development compared to children born to term with an associated risk of long-term sequelae. The higher morbidity found in children born LPT compared to their full-term (FT) counterparts is not limited to infancy, but persists through childhood, with a higher frequency of readmission and emergency department visits, a higher risk of infection, respiratory problems, and a mortality that is 2–3 times greater compared to FT infants.1
The SEN34-36 working group of the Sociedad Española de Neonatología (Spanish Society of Neonatology), in collaboration with the Asociación Española de Pediatría de Atención Primaria (Spanish Association of Primary Care Paediatrics), has developed these follow-up recommendations with the main objective of reducing the impact of prematurity in the development of children born LPT. The full text of these recommendations can be found at www.se-neonatal.es/Portals/0/Publicaciones/Protocolo_Prematuro_tardio.pdf. The secondary objectives of this work were to raise awareness in neonatologists and paediatricians of the potential sequelae of LPT birth, to establish and standardise the evaluations and/or interventions that should be performed in the LPT population, to offer tools for clinical follow-up for the early detection of developmental impairments and to facilitate the coordination of care between all involved health care providers.
JustificationThere is sufficient evidence to assert that LPT infants are at higher risk of neurodevelopmental impairment, the degree of which is inversely proportional to gestational age at birth. Multiple factors are at play that can be attributed to genetic or epigenetic causes. But in preterm infants, neurologic development from birth to the due date occurs outside the uterus and is probably disturbed by this change in environment. The period between 34 and 40 weeks’ gestation is not only characterised by an increase in brain weight but is also a critical and highly sensitive period in the maturation of brain structures, which may be affected by preterm birth.2 Other factors may also play a role: the morbidity associated with prematurity, the absence of breastfeeding (BF) and low parental socioeconomic level, described as risk factors for poor neurologic outcomes in children born LPT.1
The risk of neurodevelopmental delays is twice as high in children born LPT compared to their FT peers, and they are also more likely to exhibit impairments in motor skills, communication and personal and social functioning.3,4 The risk of a low intellectual quotient, learning disorders and difficulties with cognitive and emotional regulation is greater in children born LPT compared to those born at term.5 Late preterm birth has been associated with lower scores in episodic memory, executive functioning and overall neurocognitive functioning in adulthood. Adults who were born LPT have a lower socioeconomic status, require more social services, have a lower educational attainment and are more likely to be unemployed compared to their FT peers.6
Nutrition is a key aspect in the care of LPT infants because it has an impact on growth and development.7 The advantages of BF are even greater in LPT infants compared to FT infants. However, successful establishment of BF in this population is frequently more challenging. Immaturity is associated with increased sleepiness, weaker muscles and poorer sucking skills, with difficulties in the coordination of sucking and swallowing that predispose them to inadequate intake and their mothers to produce an inadequate milk supply.8 All of the above can lead to malnutrition, dehydration and bilirubinaemia, especially in infants born to older primiparous mothers or delivered by caesarean section.8 Their specific nutritional risk, which clinicians are often unaware of, results in higher mortality and readmission rates, 2–3 times greater compared to FT newborns in the first 15 days of life.9
The risk of delayed growth in children born moderate or late preterm is 2.5 times greater compared to children born at term.10 Undernutrition at the beginning of life has irreversible effects on the central nervous system and may lead to impaired cognitive development. On the other hand, an excessively rapid weight gain during infancy and early childhood has been associated with metabolic syndrome in adulthood.8
Respiratory morbidity in children born LPT is frequent in both infancy and the medium and long term. Neonatal respiratory complications develop in 10.5% of LPT newborns compared to 1.13% of FT newborns. Evidence from various studies suggests that administration of antenatal corticosteroids in this population could reduce the incidence of respiratory complications in the perinatal period.11 Children born LPT are at higher risk of bronchitis (RR, 1.24) and asthma (RR, 1.68), of requiring inhaled steroid medications (RR, 1.66) and of being admitted to hospital for respiratory problems in the first 2 years of life (RR, 1.99) compared to children born at term.12
Infections cause significant morbidity and mortality in children born LPT and are one of the main reasons for medical visits and hospital readmission in this population. The most frequent infections are respiratory, followed by gastrointestinal infections, and account for the highest number of medical appointments and hospital readmissions. The risk of admission due to respiratory infection in the first year of life is 3 times greater in LPT infants compared to FT infants, with higher rates of admission to intensive care units, more frequent need of intubation and mechanical ventilation and longer lengths of stay.13
Routine childhood vaccination is one of the strategies that has had the most impact on public health. There is general agreement that children born preterm, and therefore those born LPT, should follow the same vaccination schedule as their FT counterparts. Compared to FT infants, PT infants exhibit a similar response to vaccination against diphtheria, tetanus and pertussis (DTaP), poliovirus 1 and 2, and pneumococcal and meningococcal disease, but a weaker response to vaccination against hepatitis B, Haemophilus influenzae and poliovirus 3. In general, the immunogenicity of vaccines in preterm infants is low, similar to the immunogenicity in FT infants, while their safety profile is satisfactory.14
The incidence of sudden infant death is of 1.37 deaths per 1000 PT births compared to 0.69 deaths per 1000 term births. Apparent life-threatening events are also more frequent in PT infants (8–10%) compared to FT infants (≤1%).15
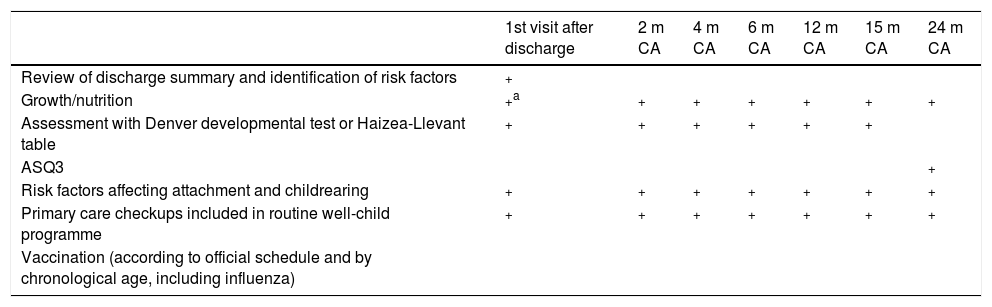
The paediatrics team in primary care centres (PCCs) plays a crucial role in the follow-up of PT infants. The purpose of the protocol for the follow-up of PT infants in PCCs is to support parents, promote healthy and positive attitudes and behaviours in children regarding health and the early detection of health problems in children, facilitating monitoring and referral, if needed. The follow-up protocol includes many of the elements of the programme for preventive care in childhood (Table 1).
Follow-up calendar from discharge to 24 months of corrected age.
| 1st visit after discharge | 2 m CA | 4 m CA | 6 m CA | 12 m CA | 15 m CA | 24 m CA | |
|---|---|---|---|---|---|---|---|
| Review of discharge summary and identification of risk factors | + | ||||||
| Growth/nutrition | +a | + | + | + | + | + | + |
| Assessment with Denver developmental test or Haizea-Llevant table | + | + | + | + | + | + | |
| ASQ3 | + | ||||||
| Risk factors affecting attachment and childrearing | + | + | + | + | + | + | + |
| Primary care checkups included in routine well-child programme | + | + | + | + | + | + | + |
| Vaccination (according to official schedule and by chronological age, including influenza) |
| 3–4 years | 5–6 years | 9 years | 11–12 years | 14 years | |
|---|---|---|---|---|---|
| Growth | + | + | + | + | + |
| ASQ3 assessment | + | + | |||
| PC checkups established in the routine well-child programme | + | + | + | + | + |
| Vaccination (according to official schedule and by chronological age, including influenza) | + | + |
Age 2–14 years.
Thus, we must be aware that while the risk of sequelae in most cases is lower compared to children born at younger gestational ages, it is still not to be overlooked. Therefore, our ultimate goal must be to be able to diagnose and treat health problems early, especially in the area of neurodevelopment, to achieve better long-term outcomes in the population born preterm (Table 2).
Grades of recommendation (based on the evidence rating scale of the Agency for Healthcare Research and Quality).
| A | There is good research-based evidence to support the recommendation. |
| B | There is fair research-based evidence to support the recommendation. |
| C | The recommendation is based on expert opinion and panel consensus. |
| X | There is evidence of harm from this intervention. |
- •
For all LPT infants, implement a specific follow-up care plan. This plan should be determined prior to discharge, taking into account the resources available in each region at the primary care and hospital levels (Table 1). Grade C recommendation.
- •
Follow-up visits should be scheduled applying the CA through age 2 years and from then on applying the chronological age through age 6 years (Table 1).
- •
Before discharge, identify the existing risk factors associated with poor neurodevelopmental outcomes before discharge, and ensure that the family understands what is needed for the care of these children.10 Grade C recommendation.
- •
All children born PT must undergo simple neurologic screening tests (such as Haizea-Llevant scale) until at least 2 years of CA during routine primary care visits or in the framework of specific hospital-based programmes for the follow-up of PT infants.16,17 Grade C recommendation.
- •
Complete the Ages and Stages Questionnaires®, 3rd edition (ASQ®, Spanish version, available at www.proyectoacuna.es) at 2 years of CA and 4 and 5 years of chronological age in all children born PT or at least those with risk factors for poor neurodevelopmental outcomes (respiratory distress, intrauterine growth restriction, symptomatic hypoglycaemia, hyperbilirubinaemia requiring phototherapy, hypoxaemia, intraventricular haemorrhage, periventricular leukomalacia, formula feeding and/or low socioeconomic status and educational attainment in the mother).4,18,19 Grade C recommendation.
- •
In patients with results below the established cut-off point for the ASQ3® in the total score or more than one domain, the evaluation should be completed in the referral hospital with specific questionnaires, such as the third edition of the Bayley Scales of Infant Development, the Modified Checklist for Autism in Toddlers in case of a suspected autism spectrum disorder or the fourth version of the Swanson, Nolan and Pelham questionnaire originally published in 1983 if attention deficit is suspected.18,20 Grade B recommendation.
- •
In case of detection of abnormalities in neurologic development, refer patient to early intervention services to minimise long-term sequelae.18,21,22 Grade B recommendation.
- •
Children born LPT in whom neurodevelopmental disorders are suspected should be evaluated by the school counselling team of their school when they start primary school for the early detection of subtle impairments. The areas assessed should include executive functioning, attention, language and social skills, as impairments in these areas can have a significant impact on academic performance.3,5 Grade B recommendation.
- •
Develop a family-centred multidisciplinary care plan in cooperation with the school and coordinated by the paediatrician. Neurologic outcomes in children born LPT must be optimised by establishing a safe and supportive family environment.23 Grade C recommendation.
- •
Human milk is the food of choice, to be fed exclusively until 6 months of CA and subsequently supplemented with other foods until age 2 years or until the mother and child so desire.24 Grade A recommendation.
- •
In infants born preterm, BF management should be specific and different from management in FT infants.7 The mother must be encouraged to express milk and supplement feeds with her own expressed milk if needed. Supplementation with formula should be used as the last recourse. Recommend kangaroo care at home after discharge, as it is an efficacious and feasible approach that promotes BF and the health and wellbeing of infants and their parents.25 Grade C recommendation.
- •
Before discharge, parents should be made aware that their child is at increased risk of feeding difficulties, hyperbilirubinaemia and dehydration. Educational interventions must focus on giving parents, and especially first-time mothers, the skills needed to identify these problems, which in some cases may require a longer hospital stay after birth.7 Grade C recommendation.
- •
Parents should be referred to breastfeeding support groups in the community. Grade C recommendation.
- •
A paediatric evaluation must be performed 24–48h after discharge, and weekly checkups at the primary care level are recommended until the child has reached 40 weeks postmenstrual age or BF has been properly established and the child exhibits adequate weight gain.7,26 Grade C recommendation.
- •
If growth is suboptimal after discharge, assess BF technique. If BF technique is correct yet weight gain continues to be less than 20g/day, the infant should be given 2 to 3 feeds a day of enriched or preterm formula through 40 weeks of postmenstrual age. Grade C recommendation.
- •
Introduce solids no earlier than 6 months of CA and once the child exhibits sufficient developmental maturity, and encourage continued BF until at least 1 year of age or beyond in combination with complementary foods.27 Grade C recommendation.
- •
Growth monitoring is an essential component of good clinical practice and is a feasible indicator of health and nutritional status8:
- ∘
Weight, length/height and head circumference must be measured in every routine paediatric checkup and entered in growth charts. Grade C recommendation.
- ∘
The use of WHO growth charts is recommended for monitoring of growth post discharge, using CA and through age 2 years.8,27 Grade B recommendation.
- ∘
- •
All PT infants must receive vitamin D and iron supplementation.7 Grade C recommendation.
- ∘
Vitamin D (prevention of osteopenia and rickets): in breastfed infants, until the introduction of complementary foods. In formula-fed infants, vitamin D supplementation may be discontinued once the infant receives at least 1L of formula fortified with vitamin D.
- ∘
Iron (prevention of iron-deficiency anaemia): supplementation in the form of ferrous salt from 2 weeks up to 6–12 months.
- ∘
- •
Administer antenatal corticosteroids to pregnant women at imminent risk of preterm labour between weeks 34+0 and 36+6 of gestation.28 Grade A recommendation.
- •
Identify PT infants at risk of respiratory morbidity based on the early neonatal period.
- •
Establish a multidisciplinary network for monitoring and follow-up of respiratory disease (pulmonologist, neonatologist, primary care paediatrician), especially for patients that had respiratory complications after birth.11,12 Grade C recommendation.
- •
Ensure the correct training and education of parents regarding their children's increased vulnerability to infection and thus the vital importance of adhering to guidelines for the prevention of respiratory diseases.29 Grade C recommendation.
- •
Individual neonatal units should consider whether to follow the recommendations for the use of palivizumab of the Sociedad Española de Neonatología or other guidelines.32,33 Grade C recommendation.
- •
Children born LPT must receive the same vaccines (same dose, schedule and route and site of administration) as children born FT.14 Grade A recommendation.
- •
Prenatal immunisation against pertussis through the administration to the pregnant mother of the Tdap vaccine is the most effective measure for prevention of whooping cough in the first months of life.34
- •
We recommend vaccination against pneumococcal disease, influenza and rotavirus in the LPT population.14,35 Grade B recommendation.
- •
Vaccination against group meningococcal disease B is also recommended in children born PT.14
- •
Preventing delays in vaccination is recommended to achieve sustained protective levels of antibodies in the early months of life, which is when these children are at highest risk.14 Position I.
- •
We recommend vaccination with the Tdap vaccine of all health care workers in contact with preterm infants.14 Position I.
- •
It is important to promote “cocooning”, vaccinating close contacts and caregivers both in the household and in neonatal units.14 Position I.
- •
Review and reinforce parents’ knowledge of the recommendations for the prevention of sudden infant death (grade A and B recommendation) and adherence to these recommendations by means of short interviews during follow-up visits.36
- •
Implement a programme for the follow-up of children born preterm between 34 and 36 weeks’ gestation (Table 1).37–39 Grade C recommendation.
- •
Design primary care strategies to prevent patients from dropping out of the developmental follow-up programme.38 Grade B recommendation.
- •
Maintain a continuing education system to ensure the correct implementation of the primary care follow-up programme. Grade C recommendation.
- •
The discharge summary will specify the hospital appointments scheduled for follow-up based on the medical history of the infant and the need for interventions that can only be delivered in hospital-based specialty units.37,39 Grade C recommendation.
- •
Assessment by the primary care paediatrician of all LPT infants within a maximum of 48h from discharge.26 Grade C recommendation.
- •
When it comes to the health care card/notebook, we recommend, in addition to filling out the data corresponding to the hospital, attaching the slips featuring the lot numbers of all administered vaccines and entering the record number for the endocrine-metabolic screening test and the results of the newborn hearing screen. The health care card/notebook is an accepted document in which to specify the schedule of future follow-up visits. Grade C recommendation.
- •
We recommend that health providers involved in the follow-up of these children have access to shared electronic health records, for which an online platform is available (Web Proyecto Acuna, www.proyectoacuna.es) with free access upon request. This platform is hosted by the Sociedad Española de Neonatología and registered with the Register of the Agencia Española de Protección de Datos (Spanish Agency for Data Protection). For as long as access is not possible, short summaries should be written to document each visit, detailing the findings of the clinical evaluation and the recommendations given based on the condition of the child.39 Grade C recommendation.
- •
In case of social or environmental risk, the PCC team should confirm the involvement of social workers and coordinate care with these services.37,39 Grade C recommendation.
The authors have no conflicts of interest to declare.
Please cite this article as: García Reymundo M, Hurtado Suazo JA, Calvo Aguilar MJ, Soriano Faura FJ, Ginovart Galiana G, Martín Peinador Y, et al. Recomendaciones de seguimiento del prematuro tardío. An Pediatr (Barc). 2019;90:318.