The finding of hypovitaminosis in pregnancy D has prompted the debate about its supplementation. The objective of the study was to measure the prevalence of hypovitaminosis D in mothers and newborns.
MethodsA one-year observational study was conducted including the measuring of vitamin D levels in mothers and in the umbilical cord blood of newborns. An analysis was made of the variables as regards maternal characteristics, delivery and sun exposure.
ResultsValues lower than 20ng/mL were found in 64.4% of 745 mothers and 41.3% of 560 newborns, and less than 30ng/mL in 88.7% and 67.1%, respectively. Mean levels were higher in summer–autumn than in winter–spring (21.73 and 13.70ng/mL in mothers and 29.04 and 20.49ng/mL in cord), and higher in the umbilical cord than in the maternal plasma. Multiple pregnancies (OR: 6.29) and non-European origin (OR: 13.09) were risk factors for maternal hypovitaminosis, while maternal supplementation (OR: 0.19), physical activity (OR: 0.57), and sun exposure (OR: 0.46) had a preventive effect.
ConclusionsThe high rates of hypovitaminosis support the policy of giving dietary supplements to newborns. The high level of hypovitaminosis found supports the extension of screening and supplementation to all pregnant women, and not only to those with risk factors. The greater difference between mothers and newborns in seasons of low sun exposure can be interpreted as a protective effect.
El hallazgo de hipovitaminosis D en el embarazo ha impulsado el debate acerca de su suplementación. El objetivo del estudio fue medir la prevalencia de hipovitaminosis D en gestantes y recién nacidos.
MétodosSe realizó un estudio observacional de un año de duración midiendo los niveles de vitamina D en madres y en sangre de cordón umbilical de recién nacidos. Se registraron variables relacionadas con las características de la madre, el parto y la exposición al sol.
ResultadosSe encontraron valores menores de 20ng/ml en el 64,4% de 745 madres y el 41,3% de 560 recién nacidos y menores de 30ng/ml en el 88,7% y 67,1% respectivamente. Los niveles medios fueron más altos en verano-otoño que en invierno-primavera (21,73 y 13,70ng/ml en madres y 29,04 y 20,49ng/ml en cordón) y mayores en el cordón umbilical que en el plasma materno. Los embarazos múltiples (OR: 6,29) y el origen no europeo (OR: 13,09) fueron factores de riesgo de hipovitaminosis materna mientras que la suplementación materna (OR: 0,19), la actividad física (OR: 0,57) y la exposición al sol (OR: 0,46) tuvieron un efecto preventivo.
ConclusionesLas altas tasas de hipovitaminosis respaldan la política de dar suplementos dietéticos a los recién nacidos. El alto nivel de hipovitaminosis encontrado apoya la extensión del cribado y suplementación a todas las embarazadas y no solamente a aquellas con factores de riesgo. La mayor diferencia entre madre e hijo en las temporadas de baja exposición solar puede interpretarse como un efecto protector.
During pregnancy, nutrition and lifestyle factors associated with vitamin D are monitored due to the key role of this vitamin in the skeletal and extraskeletal health of mothers and newborns.1,2 Despite evidence in the literature of a high prevalence of hypovitaminosis D, there is no consensus on the use of routine supplementation in the population of pregnant women due to the risk of complications associated with hypervitaminosis D.3,4 One of the points of controversy is the threshold for vitamin D levels, with a considerable difference between the cut-off point of 20ng/mL proposed for the general healthy population by the Institute of Medicine (IOM) of the United States compared to the 30ng/mL cut-off point for patients supported by national and international associations.3–7 The debate has particular implications in the paediatric population because supplementation with vitamin D during pregnancy has an impact on the health of newborns, as there is a positive correlation between maternal and neonatal vitamin D levels.8,9
The main source of vitamin D is endogenous synthesis during exposure to sunlight, while the amounts obtained from dietary sources are less important.10 However, the relative contribution of these sources varies depending on multiple factors, such as age, latitude, season, hours of sun exposure, sunscreen use, air pollution, clothing, skin pigmentation and dietary patterns.10 Furthermore, the American Academy of Dermatology advises against using exposure to ultraviolet rays without protection as a source of vitamin D.11 A more clear understanding of the role played by each of these factors in the levels of vitamin D in both pregnant women and newborns would be helpful in the development of public health policies regarding supplementation in these two populations.
The aim of our study was to establish the prevalence of hypovitaminosis D in pregnant women and umbilical cord blood samples and identify protective and risk factors.
MethodsWe conducted a cross-sectional observational study between August 1, 2012 and July 31, 2013. The sample included all pregnant women who gave birth in the Department of Obstetrics and Gynaecology of the Hospital de Zumárraga (a regional hospital in the province of Guipúzcoa, Spain). This region has a population of 100000 inhabitants, and the main towns in the area, Beasáin (latitude, 43.05N) and Zumárraga (latitude, 43.11N), received an average of 10.4–11.8MJ/m2 of solar irradiance during the study period (Euskalmet, Basque Country Meteorology Agency).12 The scope of practice of the hospital does not include high-risk deliveries, and such cases were referred directly to a tertiary care hospital.
Mothers provided written informed consent for the study. The study was approved by the Committee of Clinical Research and Ethics of Guipúzcoa. For newborns, we recorded the 1-min and 5-min Apgar scores, the weight of the placenta (g) and the birth weight, length and head circumference; for mothers, we recorded vitamin D supplementation, the skin phototype based on the Fitzpatrick scale, which ranges from I (never tans, pale white skin) to VI (always tans darkly, dark brown or black),13 physical activity on a scale of 1–4 points (1: no exercise, 2: a few walks, 3: daily walks amounting to less than 1h, and 4: daily walking for 1h or more) and sun exposure on a scale of 1–4 points (1: none, 2: <30min/day, 3: 30–60min/day and 4: >1h/day). We also recorded the month of birth, maternal geographical origin (European vs non-European), the characteristics of the birth, and the height and weight of pregnant mothers. We calculated the maternal body mass index (BMI) based on the prepartum weight.
To assess the levels of vitamin D, we measured the levels of the metabolite 25(OH)D in samples of umbilical cord blood and maternal blood collected the day after birth using a commercially available kit (Elecsys® electrochemiluminescence binding assay). The Elecsys assay performed in a Cobas analyser is calibrated by means of liquid chromatography–tandem mass spectrometry and the standard reference developed by the National Institute of Standards and Technology.14,15 We used a cut-off point of 20ng/mL to define vitamin D deficiency, although we also calculated the proportions of hypovitaminosis D applying cut-off points of 10 and 30ng/mL.
Statistical analysisWe used the Stata software, version 13.0, and set the level of statistical significance at .05. We have described the characteristics of the sample by means of absolute and relative frequencies in case of categorical variables and means and standard deviations or medians and interquartile ranges in case of continuous variables depending on whether their distribution was normal. We assessed the association between the levels of vitamin D in pregnant women and in umbilical cord blood by means of the Pearson correlation coefficient.
We performed multivariate analyses using generalised linear models.16 First, we fitted logistic regression models to explore the association of season and maternal characteristics with the probability of hypovitaminosis D in the mother. We then measured the effect of maternal vitamin D levels in the prediction of vitamin D levels in umbilical cord blood adjusting for other factors, such as season and maternal characteristics, using Gaussian family models with identity link functions.
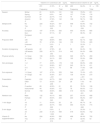
ResultsThere were 808 births during the period under study, and the mothers in 745 agreed to participate (92.2%). Due to problems with sample collection, umbilical cord blood samples were only obtained in 560 cases (75.2%). We found no statistically significant differences between the mothers that chose to participate and the mothers that declined (Appendix B, Table S1 of supplemental materials, available online) or between their newborn children (Appendix B, Table S2 of supplemental materials, available online). Table 1 presents the distribution of hypovitaminosis D based on the vitamin D levels in umbilical cord blood and maternal blood by season of the year, maternal characteristics, type of delivery, newborn characteristics and sun exposure. Table 2 shows the median and mean values of continuous variables analysed in mothers and newborns stratified by the vitamin D levels in umbilical cord blood and maternal blood. The median maternal age was 33.14 years (IQR, 30.3–35.9) and 10.7% of mothers had non-European ancestry. Only 5.8% had received some form of vitamin D supplementation, but in this group the prevalence of hypovitaminosis D decreased to 32% of the mothers. When it came to the type of delivery, 14.2% were by caesarean section, and 1.7% corresponded to multiple births. The serum levels of 25(OH)D were less than 30ng/mL in 88.7% of the mothers and 67.1% of newborns, less than 20ng/mL in 64.4% of mothers and 41.3% of newborns, and less than 10ng/mL in 26.8% of mothers and 11.1% of newborns. Maternal hypovitaminosis was significantly associated with delivery in winter or spring and non-European ancestry, younger age, little to no physical activity, a prepartum BMI of more than 30kg/m2, a dark skin phototype and low levels of sun exposure. When it came to the characteristics of the birth, maternal hypovitaminosis was significantly associated with multiple births and caesarean delivery (Table 1). We found that vitamin D levels in umbilical cord blood were significantly associated with the same variables as maternal hypovitaminosis, except for maternal prepartum maternal BMI and the type of delivery. We did not find an association between neonatal characteristics (such as the Apgar scores or the birth weight, length or head circumference) with the presence of hypovitaminosis D (Tables 1 and 2).
Distribution of hypovitaminosis in mothers according to maternal characteristics, type of delivery and sun exposure.
| Vitamin D in cord blood <20ng/mL | Maternal serum vitamin D <20ng/mL | ||||||
|---|---|---|---|---|---|---|---|
| 231 | 41.3% | N=560 | 480 | 64.4% | N=745 | ||
| Frequency | Total | Frequency | Total | ||||
| Season | Winter | 81 | 50.3% | 161 | 160 | 79.6% | 201 |
| Spring | 84 | 6.0% | 140 | 137 | 77.8% | 176 | |
| Summer | 27 | 22.7% | 119 | 75 | 41.2% | 182 | |
| Autumn | 39 | 27.9% | 140 | 108 | 58.1% | 186 | |
| P | <.001 | <.001 | |||||
| Multiple birth | No | 220 | 40.2% | 547 | 468 | 63.9% | 732 |
| Yes | 11 | 84.6% | 13 | 12 | 92.3% | 13 | |
| P | .002 | .039 | |||||
| Ancestry | European | 197 | 37.5% | 525 | 403 | 60.6% | 665 |
| Non-European | 34 | 97.1% | 35 | 77 | 96.3% | 80 | |
| P | <.001 | <.001 | |||||
| Prepartum BMI | ≤30 | 152 | 38.6% | 394 | 324 | 62.1% | 522 |
| >30 | 78 | 47.6% | 164 | 155 | 70.5% | 220 | |
| Unknown | 1 | 5.0% | 2 | 1 | 33.3% | 3 | |
| P | .059 | .029 | |||||
| Duration of pregnancy | <38 weeks | 22 | 47.8% | 46 | 38 | 64.4% | 59 |
| ≥38 weeks | 209 | 40.7% | 514 | 442 | 64.4% | 686 | |
| P | .353 | 1.00 | |||||
| Physical activity | 1–2 (low) | 156 | 45.9% | 340 | 320 | 69.7% | 459 |
| 3–4 (high) | 75 | 34.1% | 220 | 160 | 55.9% | 286 | |
| P | .006 | <.001 | |||||
| Skin phototype | 1–2 | 77 | 43.3% | 178 | 139 | 66.8% | 208 |
| 3 | 113 | 36.8% | 307 | 243 | 59.1% | 411 | |
| 4–5 | 41 | 54.7% | 75 | 98 | 77.8% | 126 | |
| P | .015 | <.001 | |||||
| Sun exposure | 1–2 (low) | 167 | 47.3% | 353 | 344 | 72.4% | 475 |
| 3–4 (high) | 64 | 30.9% | 207 | 136 | 50.4% | 270 | |
| P | <.001 | <.001 | |||||
| Presentation | Cephalic | 218 | 40.7% | 536 | 452 | 64.1% | 705 |
| Other | 13 | 54.2% | 24 | 28 | 7.0% | 40 | |
| P | .208 | .501 | |||||
| Delivery | C-section | 28 | 45.9% | 61 | 83 | 78.3% | 106 |
| Instrumental | 44 | 43.6% | 101 | 78 | 69.0% | 113 | |
| Normal | 159 | 39.9% | 398 | 319 | 60.6% | 526 | |
| P | .593 | .001 | |||||
| Newborn sex | Male | 132 | 43.1% | 306 | 257 | 63.0% | 408 |
| Female | 99 | 39.0% | 254 | 223 | 66.2% | 337 | |
| P | .343 | .398 | |||||
| 1-min Apgar | <8 | 11 | 55.0% | 20 | 20 | 66.7% | 30 |
| ≥8 | 220 | 40.7% | 540 | 460 | 64.3% | 715 | |
| P | .249 | .848 | |||||
| 5-min Apgar | <8 | 1 | 5.0% | 2 | 1 | 33.3% | 3 |
| ≥8 | 230 | 41.2% | 558 | 479 | 64.6% | 742 | |
| P | 1.000 | .289 | |||||
| Vitamin D supplementation | No | 224 | 42.6% | 526 | 466 | 66.4% | 702 |
| Yes | 7 | 20.6% | 34 | 14 | 32.6% | 43 | |
| P | .012 | <.001 | |||||
BMI, body mass index.
Distribution of hipovitaminosis D based on vitamin d levels in umbilical cord blood by maternal and newborn charactyeristics.
| Newborns (NBs) | Vitamin D in NB ≥20ng/mL | Vitamin D in NB <20ng/mL | Total | P | |||
|---|---|---|---|---|---|---|---|
| 329 | 58.8% | 231 | 41.3% | 560 | |||
| Median | IQR | Median | IQR | Median | IQR | ||
| Maternal age | 33.79 | 30.8–35.7 | 32.91 | 29.5–36.2 | 33.14 | 30.3–35.9 | .097 |
| Weight of placenta (g) | 580 | 517–665 | 600 | 530–685 | 600 | 530–675 | .130 |
| Umbilical cord pH at birth | 7.25 | 7.2–7.3 | 7.25 | 7.2–7.3 | 7.25 | 7.2–7.3 | .949 |
| Birth weight (g) | 3380 | 3047–3710 | 3420 | 3140–3670 | 3395 | 3090–3686 | .430 |
| Birth length (cm) | 49 | 48–50 | 49 | 48–50 | 49.00 | 48–50 | .665 |
| Birth head circumference* (cm) | 34.86 | 1.38 | 34.80 | 1.50 | 34.81 | 1.40 | .592 |
| Maternal blood | 265 | 35.6% | 480 | 64.4% | 745 | ||
| Median | IQR | Median | IQR | Median | IQR | P | |
|---|---|---|---|---|---|---|---|
| Maternal age | 33.41 | 31.0–36.1 | 32.82 | 29.7–35.6 | 33.11 | 30.2–35.8 | .004 |
| Weight of placenta (g) | 580 | 520–660 | 607.5 | 531–690 | 600 | 530–680 | .024 |
| Umbilical cord pH at birth | 7.25 | 7.2–7.3 | 7.25 | 7.2–7.3 | 7.25 | 7.2–7.3 | .666 |
| Birth weight (g) | 3360 | 3040–3683 | 3415 | 3141–3704 | 3400 | 3117–3700 | .142 |
| Birth length (cm) | 49 | 48–50 | 49 | 48–50 | 49 | 48–50 | .981 |
| Birth head circumference* (cm) | 34.81 | 1.35 | 34.84 | 1.43 | 34.83 | 1.40 | .777 |
IQR, interquartile range; NB, newborn.
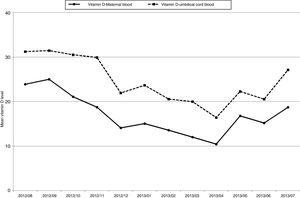
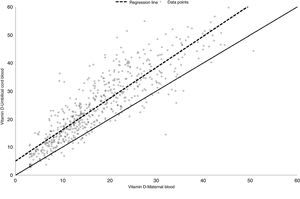
The mean vitamin D levels changed substantially between seasons. In mothers, the mean level in summer–autumn was 21.73ng/mL (standard deviation [SD], 10.0ng/mL) compared to 13.70ng/mL in winter–spring (SD, 7.4ng/mL). In newborns, the mean level changed from 29.04ng/mL in summer–autumn (SD, 12.3ng/mL) to 20.49ng/mL in winter–spring (SD, 10.4ng/mL). The levels found in umbilical cord blood were higher compared to serum maternal levels every month of the year (Fig. 1). The strong correlation between the vitamin D levels in mothers and newborns (Pearson r=0.87) can be appreciated in Fig. 2. The mere presence of hypovitaminosis D in the mother predicted hypovitaminosis D in the newborn with a sensitivity of 98.7%.
Table 3 summarises the results of the final risk prediction model for maternal hypovitaminosis D obtained by logistic regression and expressed in terms of odds ratios (ORs) with their corresponding confidence intervals (CIs). The statistically significant predictors of low vitamin D levels were multiple pregnancy (OR, 6.29) and non-European maternal origin (OR, 13.09). On the other hand, vitamin D supplementation (OR, 0.19), seasons associated with greater sun exposure (summer OR, 0.17; autumn OR, 0.42), a higher level of physical activity (OR, 0.57) and greater sun exposure (OR, 0.46) had a statistically significant protective effect. Some variables that were significant in the univariate analysis, such as caesarean delivery, maternal BMI, the weight of the placenta and maternal age, were no longer significant in the multivariate analysis and were therefore excluded from the final model.
Probability of hypovitaminosis D in mothers predicted by logistic regression based on maternal characteristics.
| Maternal vitamin D <20ng/mL | aOR | 95% CI | P | |
|---|---|---|---|---|
| Maternal age | 0.95 | (0.91; 0.99) | .016 | |
| Season | Winter | Reference | ||
| Spring | 1.05 | (0.62; 1.78) | .845 | |
| Summer | 0.17 | (0.10; 0.28) | <.001 | |
| Autumn | 0.42 | (0.26; 0.67) | <.001 | |
| Multiple birth | No | Reference | ||
| Yes | 6.29 | (1.41; 28.18) | .016 | |
| Origin | European | Reference | ||
| Non-European | 13.09 | (3.96; 43.25) | <.001 | |
| Physical activity | 1–2 | Reference | ||
| 3–4 | 0.57 | (0.40; 0.82) | .002 | |
| Amount of sun exposure | 1–2 | Reference | ||
| 3–4 | 0.46 | (0.33; 0.66) | <.001 | |
| Vitamin D supplementation | No | Reference | ||
| Yes | 0.19 | (0.09; 0.40) | <.001 |
aOR, adjusted odds ratio; CI, confidence interval.
Lastly, Table 4 presents the association of vitamin D levels in maternal blood and umbilical cord blood with the seasons of the year. The first three columns show the mean vitamin D values in mothers and newborns and their differences between the winter–spring and summer–autumn periods. While in winter–autumn the maternal mean dropped to 13.70, the difference continued to be of around 7. The other 3 columns present the results of logistic regression analysis, where the dependent variable was the level of vitamin D in newborns and the independent variables the maternal vitamin D level, the grouped season categories and their interaction. Thus, we calculated the effect of maternal levels separately for each season category. After finding a significant interaction between these variables in our model (to see the complete data, consult Appendix B, Table S3 of supplemental materials available online), we estimated that vitamin D levels in umbilical cord blood were 17.6% greater compared to maternal serum levels in births that took place in winter or spring. However, this difference was of only 11.2% in births that took place in summer or fall.
Effect of maternal vitamin D level (in ng/mL) on umbilical cord blood vitamin D levels adjusted for the season of the year.
| N | Mean vitamin D levels in maternal blood in ng/mL (SD) | Mean vitamin D levels in cord blood in ng/mL (SD) | Difference | Effect size* | Constant* | Estimate* | |
|---|---|---|---|---|---|---|---|
| Winter–spring | 301 | 13.70 (7.4) | 20.49 (10.4) | 6.79 | 17.6% | 4.618 | 7.028 |
| Summer–autumn | 259 | 21.73 (1.0) | 29.04 (12.3) | 7.31 | 11.2% | 4.618 | 7.052 |
SD, standard deviation.
Results of model 3 (Table S3 of supplemental materials available online). Statistically significant interaction between maternal vitamin D levels and grouped season category in the model where the dependent variable was the neonatal vitamin D level and the independent variables the maternal vitamin D level, the season category and their interaction.
The main finding of our study was the high proportion of hypovitaminosis D found in both mothers and newborns in the general population. We ought to highlight the large sample size of the study and that levels of 25(OH)D were measured in a cross-sectional design over a period of 1 year in a European region with a latitude of more than 40°N. Our findings confirmed the results of other studies conducted in Spain and were consistent with the prevalence of hypovitaminosis D found in pregnant women in other parts of Europe, America and Asia.17–21
The 25(OH)D levels found in newborns in our study are below the range recommended for adequate growth and development in children. Since 20% to 80% of the vitamin D in newborns comes from the mother, the levels of this vitamin may drop below the required amount in the first month of life unless an external source is provided.22 Thus, the prevalence of hypovitaminosis D of 41.3% found in newborns strongly supports the current approach of providing vitamin D supplementation.23 The high prevalence of hypovitaminosis D observed in our study can be explained by the low solar irradiance in our region and insufficient vitamin D supplementation in mothers.23
The high prevalence of hypovitaminosis D found in participants of non-European ancestry evinces marked health disparities.24,25 We understand health disparity as any difference in health status or in the access to or availability of health care associated with social, economic or ethnic characteristics.26 The higher prevalence of hypovitaminosis D found in our study could be due to the lower socioeconomic level of this collective or to the presence of barriers to their access to health care.27 However, we did not found differences in the socioeconomic data for our county that would justify this disparity,28 and pregnant women of non-European ancestry received obstetric care under the same conditions as all other pregnant women. Thus, we attribute the higher prevalence of hypovitaminosis D to the darker skin phototype, a diet with a lower content of vitamin D and that women in this population tend to use clothes that cover larger portions of their skin.26,29,30 In fact, a case of rickets diagnosed in a child of non-European ancestry was the event that prompted this study. Given the sociocultural factors that pose barriers to increased exposure to sunlight, programmes for vitamin D supplementation in pregnant women are needed to reduce disparities in the skeletal and extraskeletal health of pregnant women and children of non-European origin. Furthermore, based on the frequency of hypovitaminosis D found in the mothers and the known effects of supplementation, it seems reasonable to implement the vitamin D status screening during pregnancy followed by supplementation recommended by several scientific societies in all pregnant women, and not only those with risk factors.3,6 Although at present there is no consensus supporting supplementation in all pregnant women,3–7 the proportion of hypovitaminosis of 89.7% or 67.1%, depending on the application of a cut-off point of 30 or 20ng/mL, needs to be taken into consideration in the practice of obstetrics, as only 5.8% of women in our sample were receiving supplementation.
Other relevant findings were the association between neonatal and maternal hypovitaminosis D and that the vitamin D levels in umbilical cord blood were nearly always higher compared to the levels in maternal blood. The results of previously published studies that analysed this association have been contradictory, as some describe higher levels of 25(OH)D in umbilical cord blood compared to maternal blood,31–33 while others have found the opposite.30,34 These inconsistencies may be due to differences in the timing of collection of maternal blood samples, as levels of 25(OH)D vary between early, middle and late pregnancy and the postpartum period.29,34,35 In our study we collected the samples the day after delivery, and the high 25(OH)D levels found in umbilical cord blood could be due to a positive maternal–foetal gradient or to placental synthesis of vitamin D. This protective mechanism would guarantee adequate vitamin D levels in the foetus and newborn during a period of rapid growth.1 Thus, we propose the hypothesis that such a protective mechanism exists based on the fact that the difference is lesser in periods with higher exposure to sunlight, that is, in summer and autumn. This finding suggests that when the availability of vitamin D decreases, maternal metabolism facilitates the maintenance of vitamin D levels in the newborn, even at the expense of the needs of the mother. This could be viewed as an evolutionary advantage that would increase the probability of a successful outcome of pregnancy. From a physiological perspective, a plausible explanation is that the total vitamin D levels in cord blood are higher because cord blood contains more vitamin-D binding protein (DBP).36 The challenge resides in the interpretation of these differences in terms of the availability of vitamin D for the mother and the foetus.37 Our hypothesis is that the conversion of vitamin D between its two different forms (unbound and bound to DBP) is characterised by a neutral equilibrium under stable conditions, but under circumstances in which foetal metabolism requires greater amounts of vitamin D, the equilibrium shifts in favour of free vitamin D. Although this hypothesis needs to be corroborated by further studies, it provides a plausible explanation of the protective mechanism.
We did not find an association between vitamin D levels in maternal blood and the anthropometric characteristics of the newborn at birth. However, other authors have found an association between vitamin D deficiency and a lesser birth weight, length and head circumference in newborns.38 Since our centre is a regional hospital, it is possible that the exclusion of high-risk deliveries from our sample due to referral of these cases to the tertiary care hospital masked the extraskeletal impact of hypovitaminosis D.
We applied a cut-off point of 20ng/mL, even though different associations consider that this threshold may not offer an accurate representation of the levels actually required during pregnancy.3,5,6,31 Given the lack of consensus on the optimal levels of 25(OH)D, we used this threshold in our study because it allowed us to build models with greater discriminatory power in the assessment of risk and protective factors. With a cut-off point of 30ng/mL, the baseline probability would be of 88.7% and the differences between subgroups would disappear.
Two strengths of the study facilitate the extrapolation of its results to other populations in Southern Europe. The first one is the high participation rate in the mothers that we approached about the study. The second was that the collection of samples throughout a whole year allowed us to observe the strong influence of seasonality on the outcomes. Although the latitude of our region must be taken into account (>40°N), seasonal variation was a very relevant factor, as the lowest vitamin D levels were found in births in late winter or early spring.
The findings of our study support the routine screening of all pregnant women to make decisions regarding supplementation. Although the prevalence of hypovitaminosis D in pregnant women may vary between 67.1% and 88.7% depending on the reference range applied in its definition, it seems reasonable to propose a measure that would benefit at least two thirds of pregnant women and nearly half of all newborns.
FundingThis project was funded by the Department of Health of the Basque Country (Project number 2011111107).
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Blarduni E, Arrospide A, Galar M, Castaño L, Mar J, Grupo GOIVIDE. Factores asociados a la prevalencia de hipovitaminosis D en mujeres embarazadas y sus recién nacidos. An Pediatr (Barc). 2019;91:96–104.