Patients with achondroplasia present, in addition to disproportionate short stature, multiple manifestations that require a comprehensive approach. The present consensus of experts in Spain responds to the need to establish clear guidelines for the management of achondroplasia with the introduction of a new treatment, vosoritide.
Material and methodsA panel of six experts in achondroplasia participated in the development of the consensus. They developed a narrative review of the recommendations on achondroplasia and vosoritide treatment, which were agreed upon and adapted to the Spanish context in two subsequent meetings with a structured discussion format.
ResultsThis protocol underscores that achondroplasia requires specialised and multidisciplinary management involving expert paediatricians and specialists in paediatric endocrinology or medical genetics, in collaboration with specialists in neurology, neurosurgery, pneumology, otorhinolaryngology, rehabilitation, and orthopaedics or psychology, among others, with adequate coordination of care. This interdisciplinary team should be involved in the planning of vosoritide treatment (including the education of patients and caregivers, with management of their expectations, and their training in the practical aspects of vosoritide administration), treatment initiation and close monitoring with regular assessment of anthropometric, biochemical, functional or patient-reported variables.
ConclusionsThis protocol for the administration of vosoritide will allow standardised implementation and optimization of treatment outcomes. It also offers an opportunity to improve the management of achondroplasia in Spain through a comprehensive and interdisciplinary approach.
Los pacientes con acondroplasia presentan, además de una talla baja desproporcionada, múltiples manifestaciones que requieren de un abordaje integral. El presente consenso de expertos en España responde a la necesidad de establecer unas directrices claras para el manejo de la acondroplasia con la introducción de un nuevo tratamiento, vosoritida.
Material y métodosPara la elaboración del consenso participó un panel de seis expertos en acondroplasia. Estos desarrollaron una revisión narrativa sobre las recomendaciones en acondroplasia y el tratamiento con vosoritida, que se consensuaron y adaptaron al contexto español en dos reuniones posteriores con formato de discusión estructurada.
ResultadosEste consenso de expertos incide en que la acondroplasia requiere de un manejo especializado y multidisciplinar donde se implique, de manera coordinada a pediatras expertos, endocrinólogos pediatras o médicos genetistas, junto con especialistas en neurología, neurocirugía, neumología, otorrinolaringología, rehabilitación, ortopedia o psicología, entre otros. Este equipo multidisciplinar debe estar involucrado desde la planificación del tratamiento con vosoritida –mediante la educación al paciente y sus cuidadores, el manejo de sus expectativas o la instrucción en los aspectos prácticos de la administración– su inicio, así como en un seguimiento estrecho donde se valoren sistemáticamente variables antropométricas, bioquímicas, funcionales o aquellas comunicadas por el paciente.
ConclusionesEl presente protocolo para la administración de vosoritida permitirá la implementación homogénea y la optimización de los resultados de este tratamiento; así mismo, representa una oportunidad de mejorar el manejo de la acondroplasia en España desde un enfoque integral y multidisciplinar.
Achondroplasia is the most common form of skeletal dysplasia with disproportionate short stature, although it is considered a rare disease, as its incidence is estimated to be between 1/10 000 to 1/30 000 births.1,2 It is a genetic disorder caused by heterozygous pathogenic variants in the FGFR3 gene that encodes fibroblast growth factor receptor 3 (OMIM 134934), which inhibits endochondral bone development. Ninety-nine percent of affected patients carry the c.1138G>A pathogenic variant (NM_000142.5), and the remaining 1% carry the c.1138G>C variant (NM_000142.5). In 80% of cases, the parents are unaffected and the patient carries a de novo variant, and the change has 100% penetrance. In addition, features of the disease are not detected until the third trimester of pregnancy, resulting in delayed diagnosis.3
Achondroplasia is a complex chronic condition with multiple clinical manifestations that may or not be associated with impaired growth. The changes in endochondral ossification results in disproportionate short stature, usually with rhizomelic limb shortening (mean height, 131 cm [SD, 5.6] in men and 124 cm [SD, 5.9] in women), a characteristic phenotype with macrocephaly, bulging forehead, midface hypoplasia and trident hand and foramen magnum stenosis, the latter of which carries a risk of hydrocephalus (increased risk of sudden death in the first years of life), an increased risk of recurrent otitis that may result in hearing loss and language delays in early childhood, genu varum deformities (found in 40%–70% of patients) that may require surgical correction3,4 or lumbar hyperlordosis and spinal stenosis that may result in leg claudication and limitation of daily activities. In addition, these patients are predisposed to obesity and metabolic syndrome, which increase cardiovascular risk and reduce life expectancy.5
This collection of clinical manifestations has a negative impact on the health-related quality of life (HRQoL) of the patients and their caregivers.6 In addition, affected individuals may have self-esteem or anxiety problems (found in 50%–60% of patients) due to the social implications of their disease.7,8
Until recently, surgery has been the chief therapeutic option for these patients.9,10 This includes neurosurgical interventions, for instance for treatment of foramen magnum stenosis, or orthopaedic interventions, such as osteotomy for correction of genu varum or limb lengthening surgery. However, recent advances in the understanding of the pathogenesis of achondroplasia have paved the way for the development of treatments like FGFR3 modulators.11–13 One of them is vosoritide, who has been found efficacious in clinical trials in increasing growth velocity in children with achondroplasia.14–17
Given the complexity of the disease and its treatment, achondroplasia requires multidisciplinary management in specialised centres. For this reason, in recent years, several guidelines have been published with the aim of optimising the comprehensive management of these patients, to improve their HRQoL and to promote their autonomy and social inclusion.1,18–20 In spite of it, there is still substantial heterogeneity in the approach to the management and follow-up of patients with achondroplasia,21,22 which reflects the lack of clear and unified standards for the management of these patients and of guidelines including the use of novel therapies such as vosoritide in Spain.
In broad terms, the aim of the expert consensus presented here was to address these issues in Spain and to define the main guidelines for the multidisciplinary management of achondroplasia in order to standardise the protocol for treatment with vosoritide and its monitoring in our country.
Material and methodsThe consensus development process was carried out by a panel of 6 experts on achondroplasia and treatment with vosoritide including specialists in paediatric endocrinology, medical genetics and orthopaedics/traumatology. They conducted a narrative review of the literature and prepared a preliminary report on current recommendations for achondroplasia and treatment with vosoritide. Two meetings were held to carry out a structured review of the recommendations and reach a consensus through targeted discussions.
Preparatory workFirst of all, the experts carried out a narrative review with the aim of compiling current guidelines and consensus documents on achondroplasia, and studies on the efficacy, safety or posology of vosoritide. The collected publications provided the necessary background to develop a preliminary report that included the main recommendations extracted from the literature.
Expert meetingsThen, the experts held two meetings to reach a consensus regarding the recommendations found in the literature and their adaptation to the particular circumstances of Spain. These meetings were organised as round tables in which all experts presented their opinions, experiences and knowledge on the subject. A qualitative consensus was reached through structured and targeted questions on the recommendations proposed in the in the preliminary report.
ResultsBelow we present the main recommendations on which the panel reached a consensus regarding the care model for the administration of vosoritide and the different phases of treatment: preparation, initial evaluation and follow-up.
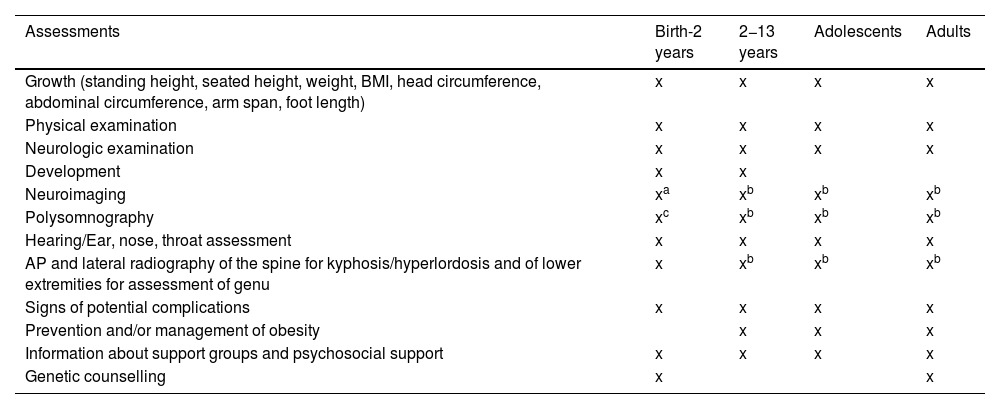
Care model for the implementation of the protocolThe complexity of the disease and of its treatment clearly call for a coordinated and specialised multidisciplinary management approach (Table 1 presents the timeline for the routine follow-up of patients with achondroplasia, adapted by Leiva-Gea et al.20) that is not always implemented. Therefore, the introduction of vosoritide offers an opportunity to initiate adequate and comprehensive follow-up in these patients. This approach allows safer delivery of treatment and rigorous assessment of its outcomes. In addition, it can improve patient care as well as patient’s autonomy and adherence to treatment.8 Thus, this protocol is intended for multidisciplinary care teams with experience in achondroplasia, ideally located in a reference centre for skeletal dysplasias.1 The care team must have a main coordinator responsible for ensuring uniform care delivery and facilitate communication between team members. Care coordinators must have proven experience in the management of achondroplasia, independently of the field in which they are specialised.1,8,23 Ideally, the multidisciplinary care team would include a specialist in medical genetics and/or a paediatric endocrinologist and/or a paediatrician expert in skeletal dysplasias in addition to specialists in the fields of paediatric neurology, neurosurgery, paediatric pulmonology, otorhinolaryngology, paediatric orthopaedics and traumatology and rehabilitation. Other specialists involved in the care of these patients include anaesthesiologists, psychologists and social workers.24 It is important that patients as well as caregivers receive psychological support from the outset and throughout follow-up. In addition, involvement of a nurse case manager is recommended to provide continuous support the family and schedule the required appointments and tests. The case manager would also be responsible for ensuring continuity of care and assist the transition from paediatric to adult care. All these specialists must have clinical experience in the management of achondroplasia and the existing resources for supporting patients and families.24,25
Timeline for the follow-up of patients with achondroplasia, adapted from the American Association of Pediatrics.
| Assessments | Birth-2 years | 2−13 years | Adolescents | Adults |
|---|---|---|---|---|
| Growth (standing height, seated height, weight, BMI, head circumference, abdominal circumference, arm span, foot length) | x | x | x | x |
| Physical examination | x | x | x | x |
| Neurologic examination | x | x | x | x |
| Development | x | x | ||
| Neuroimaging | xa | xb | xb | xb |
| Polysomnography | xc | xb | xb | xb |
| Hearing/Ear, nose, throat assessment | x | x | x | x |
| AP and lateral radiography of the spine for kyphosis/hyperlordosis and of lower extremities for assessment of genu | x | xb | xb | xb |
| Signs of potential complications | x | x | x | x |
| Prevention and/or management of obesity | x | x | x | |
| Information about support groups and psychosocial support | x | x | x | x |
| Genetic counselling | x | x |
AP, anteroposterior; BMI, body mass index.
Vosoritide is a C-type natriuretic peptide analogue indicated for use in patients with genetically confirmed achondroplasia from age 4 months25 (the indication has been authorised by the European Medicines Agency [EMA], and treatment in Spain is funded by the public health system26,27) and that can be administered until growth plates close and the growth velocity is less than 1.5 cm/year.
It is administered subcutaneously once a day. The currently available preparations are all in vials (vosoritide 0.4 mg, 0.56 mg and 12 mg powder) accompanied by a glass syringe prefilled with water for its reconstitution.25 The customary dose is of 15–30 μg/kg body weight,15 although it may vary based on the volume of distribution of the drug in relation to the weight.
Appendix B presents the recommendations regarding the presentation, injection and storage of the drug in greater detail.
Preparation for treatment initiationBefore starting vosoritide, the multidisciplinary care team should provide the necessary information to both patients and their caregivers so that they can make informed decisions about treatment. It may also be useful to have a separate conversation with the patient, adapting the language as appropriate, to establish the child’s views and understanding.24 During these conversations, it is important to discuss the motivation of the patient and caregivers for undertaking treatment with vosoritide,28 eligibility criteria, the timeframe and accepted measures of treatment response and the safety of treatment. Providers should also emphasise that vosoritide is an elective treatment and that its discontinuation would not harm the patient.24 It is important to allow sufficient time to patients and caregivers to process this information and make any relevant questions. Another aspect to consider is that patients and caregivers may have received information about vosoritide from various sources that may have elicited confusion or concern. These issues should also be addressed.24
Regarding treatment response, providers should inform that vosoritide increases linear growth (average of 1.57 cm/year, greater in male than in female patients: 1.98 vs 1.55 cm/year), and that this increase in final height may have functionally beneficial consequences.16,29 They should also clarify that the response to vosoritide may vary between patients, and that various factors may have an impact on height gain.29 Furthermore, they ought to explain that the response to treatment cannot be assessed immediately and requires repeated anthropometric evaluations for a minimum of 1–2 years after treatment initiation.16,24,29 It can be helpful to show patients specific achondroplasia growth curves so that they can discern the beneficial effect of treatment. Providers must explain that, at present, there is no evidence that vosoritide improves other areas other than linear growth,24 although it is associated with an improving trend in the disproportion between the upper and lower body segments.24,30 In terms of safety, providers must explain the type of adverse events observed in clinical trials and warn of the potential occurrence of serious adverse events that, while infrequent, have been reported in other studies.15,17,30
Initial evaluationDuring the initial visit, the coordinator will reinforce previous guidance regarding treatment expectations and provide information to the patient and caregivers on the procedure to be performed or education on how to administer the drug. Training for subcutaneous administration of vosoritide consists of a practical workshop adapted to the psychosocial characteristics of the patient and caregivers.
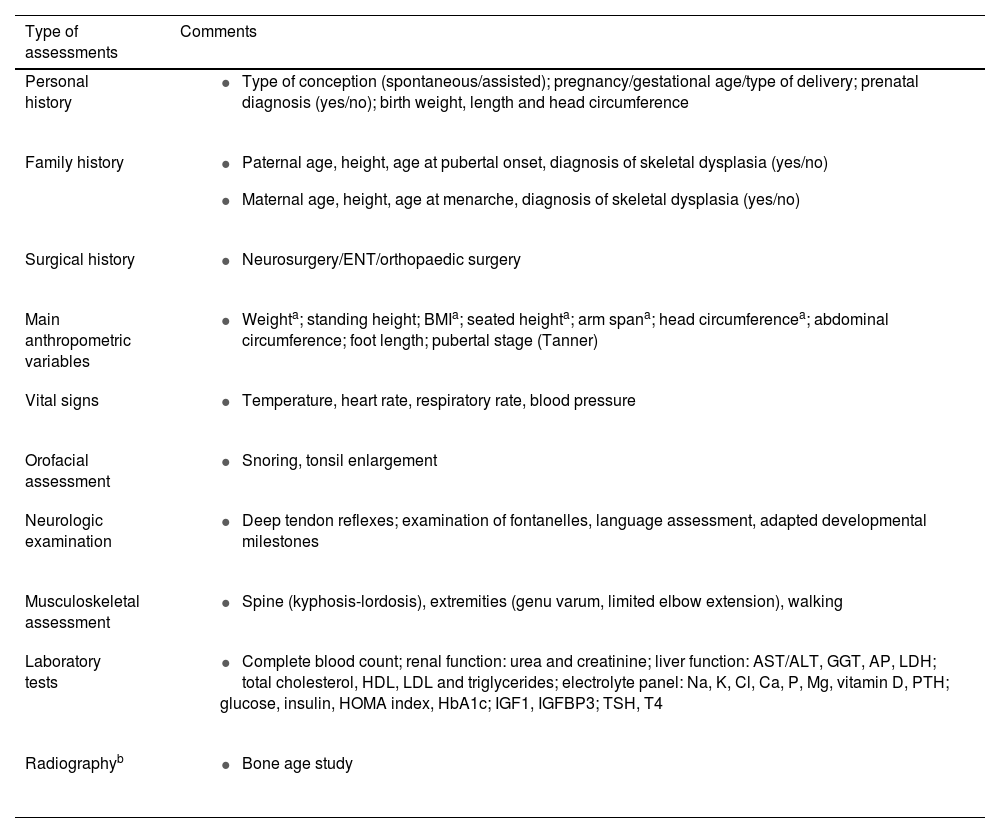
In this visit, the coordinator will also carry out a comprehensive assessment of the patient, including history-taking, verification of eligibility criteria for vosoritide and additional recommended examinations and diagnostic tests, which are detailed in Table 2. This is also the time to check whether the follow-up of the patient to date has adhered to current guidelines (Table 1).
List of assessments to be performed by main care coordinator.
| Type of assessments | Comments |
|---|---|
| Personal history |
|
| Family history |
|
| Surgical history |
|
| Main anthropometric variables | |
| Vital signs |
|
| Orofacial assessment |
|
| Neurologic examination |
|
| Musculoskeletal assessment |
|
| Laboratory tests |
|
| Radiographyb |
|
ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; BMI, body mass index; Ca, calcium; Cl, chloride; ENT, ear-nose-throat; GGT, gamma-glutamyl transpeptidase; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; HOMA, Homeostasis Model Assessment; IGF1, insulin-like growth factor 1; IGFBP-3, insulin-like growth factor binding protein-3; K, potassium; LDH, lactate dehydrogenase; LDL, low-density lipoprotein; Mg, magnesium; P, phosphorus; PTH, parathyroid hormone; TSH, thyroid-stimulating hormone.
Treatment initiation (day 0) can take place on the day of the initial visit to the coordinator or on a different day, depending on the protocols of each facility.
Prior to vosoritide administration, the nurse case manager will educate caregivers on how to prepare and inject the drug (see instructions in Appendix B). The case manager should also propose downloading the myVOXZOGO app, which allows both health care providers and caregivers to monitor adherence. This app includes information on achondroplasia and the use of vosoritide.
The nurse will record the patient's vital signs before administering the drug and 1 h after, watch for local reactions and symptoms of hypotension, and inform caregivers about possible adverse events and how to act if they occur. Hospital contact details will also be provided for reporting of any adverse events. To ensure that caregivers have become competent in the injection of the drug, they can visit the hospital two or three more days to administer the drug under supervision.
In this and all other visits, the hospital pharmacy staff will dispense the medication. In this regard, is essential that caregivers have a portable fridge or cooler to transport the medication without breaking the cold chain.
Follow-up after treatment initiation: telephone visitsWe recommend scheduling a first remote visit a week or a month after treatment initiation, depending on the needs of the patient and the protocols of the centre. In this appointment, the nurse case manager will make an assessment to ensure that treatment is going well. The nurse will address any concerns that may have arisen for the patient and caregivers and monitor adherence and adverse events.
Follow-up after treatment initiation: in-person visitsDuring the first year of treatment, patients will have visits every 3 months. At these visits, the specialist in charge of follow-up will perform a checkup and collect anthropometric measurements standardised using as reference the Neumeyer adapted growth charts for patients with achondroplasia.31,32 Changes in growth velocity and safety aspects should be rigorously monitored, and adherence and difficulties with injection technique also be considered, especially in the youngest patients.
After the first year, visits should be scheduled every 6 months. In these visits, the specialist will check whether the patient meets the growth velocity criterion and determine whether treatment should continue or not. Specifically, treatment should be discontinued if the growth velocity is less than 1.5 cm/year and/epiphyseal closure has taken place. These are indicators that the patient has no further growth potential.25
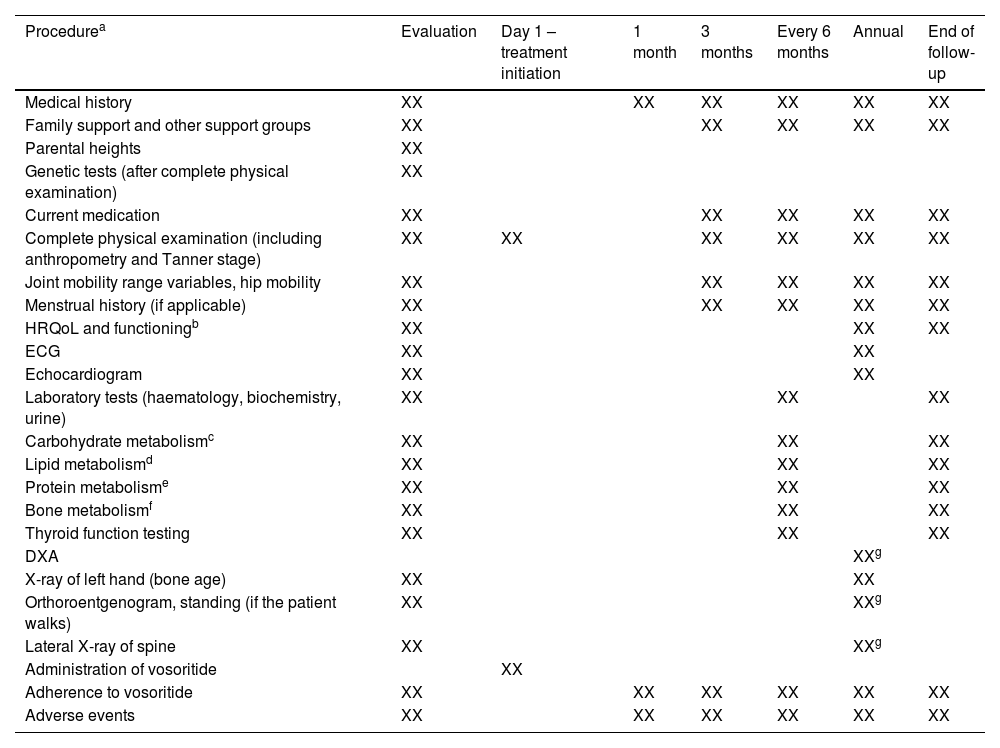
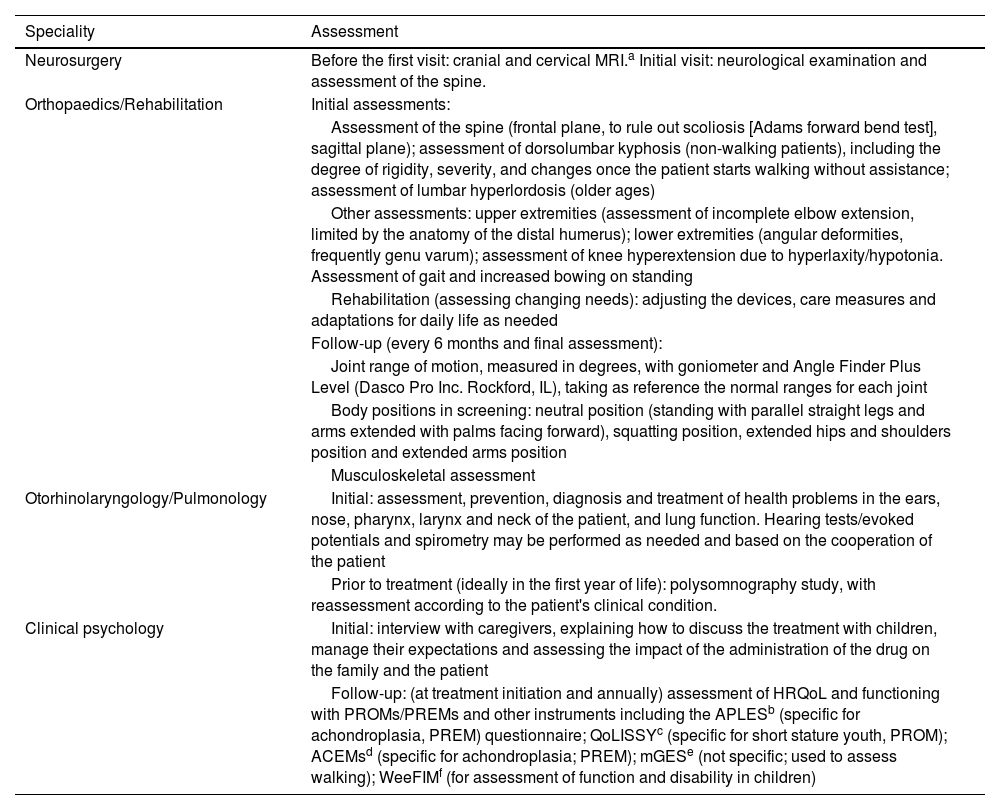
Table 3 summarises the assessments and tests performed at treatment initiation and during follow-up and their frequency. Table 4 presents the main assessments performed by the different specialists involved in the management of achondroplasia.
List of assessments and tests and their frequency at baseline and during treatment follow-up.
| Procedurea | Evaluation | Day 1 – treatment initiation | 1 month | 3 months | Every 6 months | Annual | End of follow-up |
|---|---|---|---|---|---|---|---|
| Medical history | XX | XX | XX | XX | XX | XX | |
| Family support and other support groups | XX | XX | XX | XX | XX | ||
| Parental heights | XX | ||||||
| Genetic tests (after complete physical examination) | XX | ||||||
| Current medication | XX | XX | XX | XX | XX | ||
| Complete physical examination (including anthropometry and Tanner stage) | XX | XX | XX | XX | XX | XX | |
| Joint mobility range variables, hip mobility | XX | XX | XX | XX | XX | ||
| Menstrual history (if applicable) | XX | XX | XX | XX | XX | ||
| HRQoL and functioningb | XX | XX | XX | ||||
| ECG | XX | XX | |||||
| Echocardiogram | XX | XX | |||||
| Laboratory tests (haematology, biochemistry, urine) | XX | XX | XX | ||||
| Carbohydrate metabolismc | XX | XX | XX | ||||
| Lipid metabolismd | XX | XX | XX | ||||
| Protein metabolisme | XX | XX | XX | ||||
| Bone metabolismf | XX | XX | XX | ||||
| Thyroid function testing | XX | XX | XX | ||||
| DXA | XXg | ||||||
| X-ray of left hand (bone age) | XX | XX | |||||
| Orthoroentgenogram, standing (if the patient walks) | XX | XXg | |||||
| Lateral X-ray of spine | XX | XXg | |||||
| Administration of vosoritide | XX | ||||||
| Adherence to vosoritide | XX | XX | XX | XX | XX | XX | |
| Adverse events | XX | XX | XX | XX | XX | XX |
HRQoL, health related quality of life; DXA, dual-energy X-ray absorptiometry; ECG, electrocardiogram.
Includes: total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides.
Detailed list of the assessments carried out by different specialists.
| Speciality | Assessment |
|---|---|
| Neurosurgery | Before the first visit: cranial and cervical MRI.a Initial visit: neurological examination and assessment of the spine. |
| Orthopaedics/Rehabilitation | Initial assessments: |
| Assessment of the spine (frontal plane, to rule out scoliosis [Adams forward bend test], sagittal plane); assessment of dorsolumbar kyphosis (non-walking patients), including the degree of rigidity, severity, and changes once the patient starts walking without assistance; assessment of lumbar hyperlordosis (older ages) | |
| Other assessments: upper extremities (assessment of incomplete elbow extension, limited by the anatomy of the distal humerus); lower extremities (angular deformities, frequently genu varum); assessment of knee hyperextension due to hyperlaxity/hypotonia. Assessment of gait and increased bowing on standing | |
| Rehabilitation (assessing changing needs): adjusting the devices, care measures and adaptations for daily life as needed | |
| Follow-up (every 6 months and final assessment): | |
| Joint range of motion, measured in degrees, with goniometer and Angle Finder Plus Level (Dasco Pro Inc. Rockford, IL), taking as reference the normal ranges for each joint | |
| Body positions in screening: neutral position (standing with parallel straight legs and arms extended with palms facing forward), squatting position, extended hips and shoulders position and extended arms position | |
| Musculoskeletal assessment | |
| Otorhinolaryngology/Pulmonology | Initial: assessment, prevention, diagnosis and treatment of health problems in the ears, nose, pharynx, larynx and neck of the patient, and lung function. Hearing tests/evoked potentials and spirometry may be performed as needed and based on the cooperation of the patient |
| Prior to treatment (ideally in the first year of life): polysomnography study, with reassessment according to the patient's clinical condition. | |
| Clinical psychology | Initial: interview with caregivers, explaining how to discuss the treatment with children, manage their expectations and assessing the impact of the administration of the drug on the family and the patient |
| Follow-up: (at treatment initiation and annually) assessment of HRQoL and functioning with PROMs/PREMs and other instruments including the APLESb (specific for achondroplasia, PREM) questionnaire; QoLISSYc (specific for short stature youth, PROM); ACEMsd (specific for achondroplasia; PREM); mGESe (not specific; used to assess walking); WeeFIMf (for assessment of function and disability in children) |
ACEMs, Achondroplasia Child Experience Measures; APLES, Achondroplasia Personal Life Experience Scale; mGES, modified Gait Eficacy Scale; PREM, patient-reported experience measure; PROM, patient-reported outcome measure; QoLISSY, Quality of Life of Short Stature Youth; WeeFIM: Functional Independence Measure for Children.
There is currently no consensus on the performance of annual radiographs to assess bone age throughout the follow-up. Moreover, there are limitations to their interpretation, as the available reference that is used (the Greulich and Pyle atlas) does not include subjects with achondroplasia, so the natural history in these patients is unknown. However, there is the possibility of conducting these assessments on a regular basis for research purposes, even if they do not have a direct impact on clinical decision-making.
In short, this consensus expert was motivated by the urgent need of developing a protocol for the management of achondroplasia after the introduction of a novel treatment, vosoritide, authorised in 202133 and funded by the Spanish public health system for patients starting from age 4 months.27 Thus, this is one of the first protocols, to our knowledge, developed in this new context.24,34
In addition, it addresses the need to establish optimised and standardised criteria to monitor treatment and assess outcomes of vosoritide therapy in clinical practice. This is crucial, as there are limitations to the evidence from clinical trials at the present time: followup duration of less than 10 years or lack of data on response-dependent variables.13,15–17,24,30 In addition, there is no knowledge of the potential impact of vosoritide on non-anthropometric outcomes or whether tachyphylaxis can develop in the long term.
Broadly speaking, this protocol stresses the importance of managing achondroplasia with a specialised and multidisciplinary approach, in line with the current international consensus.1,8,18 The management of achondroplasia should involve coordinated care delivery by specialists in different fields (medical genetics, paediatric endocrinology and/or paediatrics as well as paediatric neurology, paediatric pulmonology, neurosurgery, otorhinolaryngology, rehabilitation and orthopaedics, among others) taking into account recently published practical considerations for the use of vosoritide.1,8,18,24 In agreement with the current international consensus, this consensus document highlights the importance of patient and caregiver education by the multidisciplinary care team, with particular emphasis on discussing expectations regarding the effects of vosoritide and practical aspects of vosoritide administration and treatment monitoring. This was also emphasised in the article published recently by Semler et al.24
The initial assessment and follow-up of the patient must be comprehensive and requires a coordinated multidisciplinary care team. It must include an anthropometric evaluation, biochemical and functional tests and assessments of patient-reported variables. It also requires very close monitoring, with a higher frequency of visits and assessments in the first year of treatment, as specified in the international consensus.1 Follow-up must continue until the end of treatment.
In addition to the implementation of these recommendations, there are several initiatives that could advance our understanding of achondroplasia and of the impact of vosoritide on this disease. These include the establishment of registers for standardised collection of data on outcome variables, as well as the promotion of biomedical research with the creation and establishment of achondroplasia-specific biobanks for markers of bone metabolism in blood or urine specimens collected during followup.
ConclusionThis protocol for the administration of vosoritide for treatment of achondroplasia will help optimise treatment outcomes in real-world practice. It also offers an opportunity to improve the management of achondroplasia in Spain through a comprehensive and interdisciplinary approach and to gain more detailed knowledge of the impact of the treatment of achondroplasia in relation to both growth and other clinical manifestations.
FundingThis document was written by an independent medical writer, a service provided by the Nueva Investigación agency and funded by BioMarin Pharmaceutical Inc.
Conflicts of interestThe authors have received funding as consultants from BioMarin for the performance of this study.
ACB has received fees as a speaker from BioMarin and is involved in clinical trials run by BioMarin and QED Therapeutics.
IRG has received fees as a speaker from BioMarin.