Fat and fat-soluble vitamin (FSV) malabsorption is frequent in individuals with cystic fibrosis (CF) and exocrine pancreatic insufficiency (EPI). Consensus guidelines1,2 recommend FSV supplementation for patients that receive this diagnosis. Traditional supplementation may call for the ingestion of four or more units of medication a day, which poses a barrier to adherence. A new formulation (AquADEKs®) has been available in recent years that contains FSVs, water-soluble vitamins and antioxidants in doses specifically conceived for patients with CF (Table 1). All its preparations (liquid suspension, chewable tablets and soft gel capsules) have a micellar structure with a hydrophilic outer layer and a lipophilic inner layer that houses the FSVs, facilitating their absorption. Thus, our expectation was that post-intervention blood levels would remain within the normal range.
Composition, dosage and preparation of AquADEKs® according to patient's age.
| Preparation | Pediatric liquid | Chewable tablet | Soft gel capsule | |||
|---|---|---|---|---|---|---|
| Age (years) | 0–1 | 1–3 | 4–10 | >10 | 4–10 | >10 |
| Daily dose | 1mL | 2mL | 2 tablets | 4 tablets | 1 capsule | 2 capsules |
| Composition | ||||||
| Vitamin A (IU)≥87% as beta-carotene | 5751 | 11,502 | 18,167 | 36,334 | 18,167 | 36,334 |
| Vitamin D3 (IU) | 400 | 800 | 800 | 1600 | 800 | 1600 |
| Vitamin E (IU) as alpha-tocopherol | 50 | 100 | 100 | 200 | 150 | 300 |
| Vitamin K1 (mg) | 0.4 | 0.8 | 0.7 | 1.4 | 0.7 | 1.4 |
| Vitamin C (mg) | 45 | 90 | 70 | 140 | 75 | 150 |
| Thiamine (mg) | 0.6 | 1.2 | 1.5 | 3 | 1.5 | 3 |
| Riboflavin (mg) | 0.6 | 1.2 | 1.7 | 3.4 | 1.7 | 3.4 |
| Niacin (mg) | 6 | 12 | 10 | 20 | 10 | 20 |
| Vitamin B6 (mg) | 0.6 | 1.2 | 1.9 | 3.8 | 1.9 | 3.8 |
| Folic acid (μg) | 200 | 400 | 200 | 400 | ||
| Vitamin B12 (μg) | 12 | 24 | 12 | 24 | ||
| Biotin (μg) | 15 | 30 | 100 | 200 | 100 | 200 |
| Pantothenic acid (mg) | 3 | 6 | 12 | 24 | 12 | 24 |
| Vitamin E (mg) as mixture of tocopherols | 15 | 30 | 30 | 60 | 80 | 160 |
| Zinc (mg) | 5 | 10 | 10 | 20 | 10 | 20 |
| Selenium (μg) | 10 | 20 | 75 | 150 | 75 | 150 |
| Coenzyme Q10 (mg) | 2 | 4 | 10 | 20 | 10 | 20 |
| Sodium (mg) | 10 | 20 | 10 | 20 | 10 | 20 |
The aim of this study was to assess the efficacy of AquADEKs® in maintaining the serum levels of FSVs of children and adults with CF and EPI within the normal range, analysing the serum levels of FSVs at the time the administration of the new formulation was initiated, when the patients were receiving FSV supplementation with traditional formulations (pre intervention), and 3–6 months after starting supplementation with AquADEKs® (post intervention).
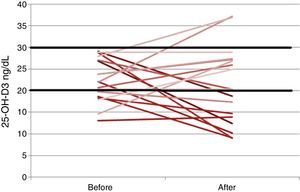
We conducted a prospective quasi-experimental study. Between September 2012 and July 2013, 31 patients with CF and EPI that were receiving FSV supplementation were included in the study; 21 were male, the mean age was 10.7±9.8 years, 12 were homozygous for Phe.508del with a mean force expiratory volume in the first second (FEV1, % over the vital capacity) of 82.8±22.2. Comparing the pre- and post-intervention periods, the mean ingestion of medication units was 3.2±1.4 versus 2±1 (P=.045); the serum levels of vitamin A (retinol), 34.04±11.7μg/dL versus 34.2±10.1μg/dL, remained stable (P=.90); the vitamin E (alpha-tocopherol) levels, 838.5±272.6ng/dL versus 1005.1±289.9ng/dL (P=.02), were higher post intervention, and the vitamin D (25-OH-D3) levels, 29.6±11.4ng/mL versus 22.6±8.5ng/mL (P=.002), were lower post intervention. Prothrombin time was normal in every case, 11.69±0.53s pre intervention compared to 11.66±0.63s post intervention, a difference that was not statistically significant.
We found two scientific studies3,4 published in the literature up to date in which this product was used in patients with CF and EPI.
When it came to vitamin A, our findings were consistent with those of the study in 11 patients by Sagel et al.,3 in which the serum concentrations of retinol remained stable and within the normal range after supplementation with AquADEKs® (pre, 39 [32–48]μg/dL vs post, 39 [35–53]μg/dL). Another study conducted by Moen et al.4 on 30 children found a significant increase in serum retinol after using AquADEKs® (pre, 31.46±8.5μg/dL vs post, 37.18±8.5μg/dL; P=.04), with concentrations within the normal range.
As for the increase in serum vitamin E concentrations, our results were similar to those of the studies by Sagel et al.3 and Moen et al.,4 which found post-intervention increases in vitamin E serum levels that did not reach statistical significance.
As for vitamin D, most patients in the study by Sagel et al.3 had insufficient levels of this vitamin (<30ng/dL) before and after the treatment. In the study by Moen et al.4 the percentage of insufficiency was slightly higher than the one in our study (50%), but deficiency was lower in theirs (25%). Since optimum levels of vitamin D were not achieved (Fig. 1) patients needed supplementation with an additional medication unit, but the number of medication units per day was still lower than under traditional supplementation (2 vs 5). To meet the current recommendations for vitamin D supplementation in patients with CF, a new formulation of AquADEKs® with a higher dose of vitamin D has been introduced recently that aims at achieving adequate concentrations of this vitamin.
One limitation of our study is that the sample size was determined by convenience sampling, with the study including the first 31 patients that met the inclusion criteria. The power was high enough for the observed decrease in vitamin D levels and the increase in vitamin E levels, and was low for the vitamin A results, although the values that we obtained lead us to believe that the clinical differences are not important or relevant.
Adherence to treatment was good, with good tolerance, and we did not observe any adverse reactions to the new formulation.
Supplementation with AquADEKs® makes multivitamin treatment easier, helps improve quality of life and facilitates adherence to treatment in patients with CF. While using this product, some patients required additional vitamin D supplements and others had high levels of vitamin E.
FundingThis study received 12,000€ of funding from Praxis Pharmaceutical, S.A.
We want to thank nurses Diana San Miguel and María Concepción Rodríguez and nurse assistant Carmen Espallargas for their collaboration in this study; as well as Javier Zamora and Alfonso Muriel, from the Biostatistics Department, Itziar de Pablo and María Ángeles Galvez, from the Ethical Committee for Clinical Research (Comité Ético de Investigación Clínica [CEIC]), and Carmen Santiuste, Óscar Pastor, Diana Cuesta and Carmen Bayón from the Biochemistry Laboratory of the Hospital Universitario Ramón y Cajal.
Please cite this article as: Garriga M, Horrisberger A, Lamas A, Ruiz de Valbuena M, Suárez L. Valoración de la utilidad de un suplemento multivitamínico estándar diseñado para pacientes con fibrosis quística. An Pediatr (Barc). 2015;83:277–279.
Previous presentation: this study was presented as an oral communication under the title “Simplificación del tratamiento multivitamínico en la fibrosis quística: ¿una solución para nuestros pacientes?” at the XII Congreso Nacional de Fibrosis Quística; November 14–16, 2013; Palma de Mallorca, Spain.