To evaluate comfort and noise intensity using the COMFORT scale in infants who receive respiratory support with a helmet interface.
Patients and methodsAn observational descriptive study was conducted on all infants (1–12 months of age) admitted to a PICU from November 1st 2013 to March 31th 2014 and who received non-invasive ventilation with a helmet interface. Tolerance to the interface was assessed by use of the COMFORT scale. The intensity of the noise to which the infants were exposed was measured with a TES1350A HIBOK 412 sound-level metre. Three measurements were made every day.
ResultsTwenty-seven patients with bronchiolitis (median age: 54 days; range: 10–256) were included. Median COMFORT score in the first day was 21 points (14–28). An increase in patient comfort was found with a gradual decrease in the scores, with a maximum reduction of 22% from the first hours (score of 22) to the fifth day (score of 18). The minimum sound intensity registered was 42dB, and the maximum was 78dB. Background noise intensity was associated with noise intensity in the helmet. No differences were observed in COMFORT score and noise intensity between ventilator devices.
ConclusionsHelmet interface was well tolerated by infants. COMFORT score results are an indicator that infants were comfortable or very comfortable. The measured noise intensity was in the safe range permitted by World Health Organization.
Evaluar el grado de bienestar y el nivel de ruido en lactantes que reciben asistencia respiratoria con interfase tipo helmet.
Pacientes y métodoEstudio analítico, observacional y descriptivo en el que se incluye a todos los lactantes (entre 1 y 12 meses de edad) con helmet ingresados en una UCIP entre el 1 de noviembre del 2013 y el 31 de marzo del 2014. Para la valoración del bienestar se utilizó la Escala de Confort Pediátrica (ECP). Los niveles de ruido fueron medidos con el sonógrafo HIBOK 412. Se realizaron mediciones 3 veces al día.
ResultadosSe incluyó a 27 pacientes con bronquiolitis (edad mediana 54 días; rango: 10–256). La puntuación mediana de ECP en el primer día fue de 21 puntos (rango: 14–28). Se observó una mejoría en el bienestar objetivado por una disminución progresiva de las puntuaciones, con una reducción máxima del 22% desde las primeras horas (puntuación de 23) al quinto día (puntuación de 18). La cifra mínima de ruido interno fue de 42dB, la máxima fue de 78dB. Las cifras de ruido externo se correlacionan con las de ruido interno tomadas en el mismo momento. No se observaron diferencias en el grado de bienestar del paciente, ni en el ruido en función del tipo de dispositivo de ventilación empleado.
ConclusionesEl helmet es una interfase bien tolerada. La puntuación COMFORT obtenida permite mantener a los niños con un grado entre cómodo y muy cómodo. Los niveles de ruido medidos se encuentran dentro del rango máximo de ruido permitido por la Organización Mundial de la Salud.
In recent years, the use of noninvasive ventilation (NIV) in acute paediatric care has been spreading and increasing in neonatal and paediatric intensive care unit (PICU) settings.1–3 The use of NIV reduces length of stay and hospitalisation costs while improving patient comfort.3,4
Helmets are one of the types of interface whose use in infants has been increasing in recent years. The interface is based on an inflatable plastic structure shaped as an astronaut's helmet that contains the entire head and neck of the child without any contact with the face. It offers several advantages, such as fewer leaks due to incorrect fitting of the interface or opening of the mouth, prevention of damage to the nasal mucosa, and allowing modifications to fit the heads of younger children, as well as a high level of humidification and continuous eye contact with the patient.5
There are also disadvantages to the helmet interface, such as increased difficulty in the management and securing of central or scalp vein catheters. It also cannot guarantee the effective delivery of nebulised medication. It is necessary to avoid excessively high temperatures inside the helmet. The helmet may make patients feel claustrophobic. Another limitation is that the face of the child cannot be freely accessed without loss of pressure; furthermore, since the dead space in helmets is greater, it requires a high flow (>30lpm) to reduce carbon dioxide rebreathing, and this flow rate could be bothersome to the child.5 Some of these drawbacks may cause discomfort in paediatric patients.
There are few studies on the helmet interface, and those that have been conducted have provided contradictory evidence as it concerns noise and patient comfort. Some studies suggest that the noise level is higher inside helmets compared to other interfaces, which may lead to discomfort in the child.6,7
The objectives of our study were to assess the comfort level of infants fitted with a helmet interface in the PICU and the noise level both inside and outside the helmet.
Materials and methodsWe designed an observational prospective study to analyse the comfort and noise levels associated with the use of the helmet interface in infants admitted to a PICU over a 5-month period (November 2013 to March 2014).
The study was approved by the Comité Ético de Investigación Clínica Regional (regional clinical research ethics committee). Infants were admitted to the study upon receipt of informed consent by the parents.
We included patients diagnosed with bronchiolitis and admitted to the PICU requiring continuous positive airway pressure (CPAP) based on the criteria of the Respiratory Working Group of the SECIP8 by consecutive sampling. In our PICU, helmets are the only type of interface used to deliver CPAP to infants. Prior to the beginning of the study, we explained its purpose to the nursing staff and provided instructions on how to collect the data.
We collected data on demographic variables, patient comfort and noise level inside and outside the helmet, and the flow generator used (Vision®, Dräger CF800® or others).
The scale used to measure the level of comfort of patients was the COMFORT-B scale,9,10 adapted from the COMFORT scale originally developed by Ambuel et al.11 for the unobtrusive assessment of PICU patients receiving mechanical ventilation. The COMFORT-B scale can be used in patients that are not intubated and assesses six behavioural parameters (alertness, agitation, crying, physical movement, muscle tone and facial tension) and two physiological parameters (blood pressure and heart rate). The final score ranges between 8 and 40 points, with a score of 8–16 indicating that the patient is very comfortable, a score of 17–26 that the patient is comfortable, and a score of 27–40 that the patient is uncomfortable or very uncomfortable. Thus, it could be considered a “discomfort” scale, as higher scores correspond to lesser comfort in the patient. Since no paediatric scales have been developed specifically for NIV, we decided to use the COMFORT-B scale, as it is the one most used internationally in the paediatric population and has been adapted from the only scale validated for children receiving mechanical ventilation.
A previously calibrated HIBOK 412 sound level metre (HIBOK Co, China) was used to measure the noise level inside and outside the helmet during the day and night shifts.
Data collection proceeded as follows: infants with helmet interfaces were observed for 2min and evaluated by means of the COMFORT scale.
We have expressed continuous variables as means±standard deviations, minimum and maximum, and categorical variables as absolute and relative frequencies. We compared variable means between groups (sex/type of device used) using the two-sample Student Welch t test. We performed intraindividual comparisons between two different measurements (different times: morning–afternoon, morning–night, afternoon–night; or inside and outside noise) by means of the paired-sample Student t test for paired samples. We analysed the association between categorical variables by means of the chi-square test for independence. The level of statistical significance was set at P<.05.
ResultsThe study sample consisted of 27 children (62.9% male). The median age was 54 days (range, 10–256 days). All participants had received a diagnosis of bronchiolitis. Thirty percent were younger than 30 days.
We included all patients that met the criteria to initiate NIV with CPAP during the period under study.
The median length of stay in the PICU was 6 days (range, 2–33 days). The median duration of ventilation with a helmet interface was 2 days (range, 1–5 days).
The flow generator used was a Vision® device in 13 (48.1%) patients, a Dräger CF800® in 11 (40.7%) and other devices in the remaining 3 (11.1%). There were no statistically significant differences in the duration of NIV with the helmet interface based on the device used.
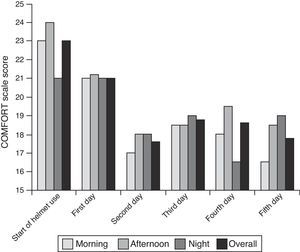
As can be seen in Fig. 1, there were no differences in comfort level based on the time of day that patients first joined the study. We also found no association between the average comfort level during the first day of helmet use and the time of day the interface was fitted on the patient.
The median comfort level at the time the helmet was fitted on the patients as measured by the COMFORT scale was 23 points (range, 12–31 points). The median comfort level score on the first day was 21 points (range, 14–28). The evolution of the average comfort level can be seen in Fig. 1. Children were comfortable with the helmet interface irrespective of age, sex, type of flow generator used and time of day that comfort was assessed with the scale. The median score in the COMFORT scale on the second day was 18 points (range, 16–27 points).
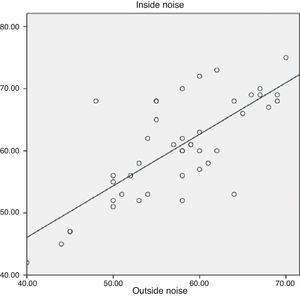
The median noise level inside the helmet was 60.5dB (range, 42–78dB) and the median noise level outside the helmet was 60.0dB (range, 52–78dB). The difference between the noise level inside and outside the helmet was not statistically significant. There was a positive correlation between both measurements (r2=0.557; P<.000) (Fig. 2).
There were no statistically significant differences between the noise level inside and outside the helmet depending on the time of day or the type of flow generator used.
DiscussionThe efficacy of the helmet system, its quick and easy fitting, greater flexibility and potential to improve patient comfort, contribute to its increasing use in PICUs.3 Our results confirm that the helmet interface is adequately comfortable for very young infants. The measured noise levels were within the range recommended by the WHO, with very small differences between the inside and outside of the helmet.
There are few studies on the level of comfort associated with the use of the helmet interface, although there are studies that have compared this interface with others used for NIV, such as oronasal, nasal and full-face masks.
The COMFORT9 scale is an unobtrusive method for assessing anxiety in PICU patients receiving mechanical ventilatory support. The use of a tool to assess pain and improve comfort in children is an attempt to avoid subjectivity. Data obtained using the COMFORT scale are used to guide clinical care, especially as it concerns pain and comfort measures, and these data directly depend on the skills of the individual applying the scale. Thus, it is essential that all staff that use the scale are trained the same way to ensure reliability and consistency, so that the same results are obtained regardless of who is applying it.10 The COMFORT scale is the tool most widely used in paediatric patients worldwide, and is the only one validated for its use in children receiving mechanical ventilation.11 No scale has been validated specifically for NIV, so the results of our study show that children are comfortable irrespective of sex, age or the time of helmet placement and the start of the study (Fig. 1). Patients were the least comfortable during the first measurement, which is probably due to the preceding clinical situation rather than the interface itself, and we observed a subsequent increase in comfort level that could have been related to clinical improvement resulting from treatment with CPAP.
There have also been few studies examining noise exposure in relation to NIV, and even fewer specifically focused on the use of helmet interfaces. Some studies have found that the noise level inside the helmet is higher than in other interfaces.6,12 The average noise level measured in our study was lower than the levels reported by similar studies, which ranged between 69.9 and 94dB.6,12–15
Based on the WHO standards, noise levels below 70dB do not harm the human body; however, a level above 85dB can already be detrimental to human health.6 In our study, noise levels ranged approximately from a minimum of 51dB to a maximum of 75dB, so we can rule out harm by exposure to excessive noise when the helmet interface is used.
We also observed that the level of noise inside and outside the helmet were the same. It seems logical to assume that if the noise level outside the helmet were lower, the noise level would also decrease inside.
The main limitation of our study is its small sample size, as we had to limit the period of data collection to 5 months. Another limitation involves the collection of data by different individuals, as the study was underway 24h a day and the data were collected by the nurses that were working in the unit at each given time. While the nurses were adequately trained beforehand, this may have resulted in interrater variability. We have concluded that the helmet is a well-tolerated interface. The COMFORT scores obtained in the study show that children can stay within the comfortable to very comfortable range, and no children scored in the uncomfortable or very uncomfortable range. The noise levels recorded both inside and outside the helmet reached a maximum of 78dB, and thus were below the maximum allowed by the WHO.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Medina A, Alvarez Fernández P, Rey Galán C, Álvarez Mendiola P, Álvarez Blanco S, Vivanco Allende A. Confort y nivel de ruido en ventilación no invasiva con interfase helmet en lactantes. An Pediatr (Barc). 2015;83:272–276.