To describe risk factors of bronchopulmonary dysplasia in very preterm infants in the first weeks of life.
Material and methodsRetrospective cohort study of preterm infants ≤ 32 weeks of gestational age and birth weight ≤ 1500 g. A multivariate logistic regression analysis was performed to identify independent risk factors for bronchopulmonary dysplasia in the first weeks of life.
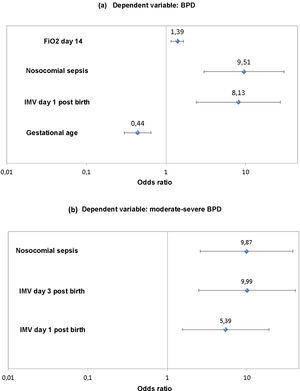
ResultsA total of 202 newborns were included in the study (mean gestational age 29.5 ± 2.1 weeks), 61.4% never received invasive mechanical ventilation. The incidence of bronchopulmonary dysplasia was 28.7%, and 10.4% of the patients were diagnosed with moderate-severe bronchopulmonary dysplasia. Bronchopulmonary dysplasia was independently associated with gestational age (P < 0.001; OR = 0.44 (95% CI = 0.30–0.65)), the need for mechanical ventilation on the first day of life (P = 0.001; OR = 8.13 ((95% CI = 2.41–27.42)), nosocomial sepsis (P < 0.001; OR = 9.51 ((95% CI = 2.99–30.28)) and FiO2 on day 14 (P < 0.001; OR = 1.39 ((95% CI = 1.16–1.66)). Receiving mechanical ventilation at the first day of life (P = 0.008; OR = 5.39 ((95% CI = 1.54–18.89)) and at the third day of life (P = 0.001; OR = 9.99 ((95% CI = 2.47–40.44)) and nosocomial sepsis (P = 0.001; OR = 9.87 ((95% CI = 2.58–37.80)) were independent risk factors for moderate-severe bronchopulmonary dysplasia.
ConclusionsGestational age, mechanical ventilation in the first days of life and nosocomial sepsis are early risk factors for bronchopulmonary dysplasia. The analysis of simple and objective clinical data, allows us to select a group of patients at high risk of bronchopulmonary dysplasia in whom it could be justified to act more aggressively, and shows areas for improvement to prevent its development or reduce its severity.
Describir los factores de riesgo de displasia broncopulmonar en las primeras semanas de vida en recién nacidos muy prematuros.
Material y métodosEstudio observacional de cohortes retrospectivo en recién nacidos ≤32 semanas y ≤ 1500 gramos. Se realizó un análisis multivariante de regresión logística para identificar factores de riesgo independientes en las primeras semanas de vida.
ResultadosSe incluyeron 202 recién nacidos con una edad gestacional media de 29,5 ± 2,1 semanas. El 61,4% de los pacientes no recibió ventilación mecánica invasiva. El 28,7% fue diagnosticado de displasia broncopulmonar, y el 10,4% de displasia broncopulmonar moderada-grave. La edad gestacional (p < 0,001; OR = 0,44 (95%IC = 0,30–0,65)), la ventilación mecánica en el día 1 (p = 0,001; OR = 8,13 ((95%IC = 2,41–27,42)), la sepsis nosocomial (p < 0,001; OR = 9,51 ((95%IC = 2,99–30,28)) y la FiO2 en el día 14 (p < 0,001; OR = 1,39 ((95%IC = 1,16–1,66)) fueron los factores de riesgo independientes de displasia broncopulmonar. La ventilación mecánica el día 1 (p = 0,008; OR = 5,39 ((95%IC = 1,54–18,89)) y 3 de vida (p = 0,001; OR = 9,99 ((95%IC = 2,47–40,44)) y la sepsis nosocomial (p = 0,001; OR = 9,87 ((95%IC = 2,58–37,80)) se asociaron al desarrollo de displasia broncopulmonar moderada-grave.
ConclusionesLa edad gestacional, la ventilación mecánica en los primeros días de vida y la sepsis nosocomial son factores de riesgo precoces de displasia broncopulmonar. El análisis de datos clínicos sencillos y objetivos nos permite seleccionar a un grupo de pacientes con alto riesgo de desarrollar displasia broncopulmonar en el que podría estar justificado actuar de forma más agresiva, y nos muestra áreas de mejora para prevenir su desarrollo o disminuir su gravedad.
Bronchopulmonary dysplasia (BPD) is a chronic pulmonary disease that affects approximately 50% of preterm neonates delivered before 28 weeks of gestation and 30% of those delivered before 32 weeks.1,2 The mortality is higher in infants that develop BPD,3 and those that survive are at increased risk of pulmonary and cardiovascular disease and, above all, neurodevelopmental sequelae, all of which are associated with a poorer quality of life and an increased use of health care resources.4–6
The pathophysiology of BPD is multifactorial and has yet to be fully elucidated.7 At its core is preterm birth,8 on which various prenatal and postnatal factors act to modulate the baseline probability of developing the disease.9 Although multiple therapies have been investigated to prevent or treat BPD, a safe and effective treatment is not yet available, and evidence is mounting that there is a window of opportunity for treatment that far predates the clinical diagnosis of BPD.10 Higgins et al.9 proposed that strategies aimed at preventing BPD should be implemented prenatally or in the first days post birth, mainly around the first week of life. Such interventions have been found to contribute to reducing neonatal mortality and some complications associated with prematurity.11,12 However, they have not resulted in a significant reduction in the incidence of BPD. The increased survival of extremely preterm infants, in whom BPD is more prevalent, could partly explain these results, but they also reflect our inability to prevent the development of BPD.13,14
Thus, identifying neonates at higher risk of BPD is one of the present challenges for neonatology and has been subject to multiple studies15 with 2 main aims. The first, at the clinical level, is to enable the identification of patients in whom the benefits of currently available treatments outweigh their risks.16 The second, at the level of research, is to allow the adequate selection of candidates for new treatments in the context of clinical trials.
The aim of our study was to establish the incidence, clinical characteristics and risk factors of cases of BPD and moderate-to-severe BPD in a contemporary sample of very preterm infants, analysing prenatal variables and neonatal outcomes in the first weeks post birth.
Material and methodsStudy design and sampleWe conducted a retrospective observational cohort study in the level IIIC neonatal intensive care unit (NICU) of a public hospital in Spain. We included infants born preterm at or before 32 weeks of gestation with birthweights of 1500 g or less in this hospital and admitted to the NICU between January 1, 2013 and August 30, 2020. We excluded infants with major congenital malformations or deceased before 28 days post birth. After applying the inclusion and exclusion criteria, we obtained a sample of 202 patients, which was large enough to estimate the incidence of BPD with a confidence of 95% and a precision of +/–6.5%, and to detect statistically significant relative risk (RR) values of 1.65 or greater associated with a risk factor expected in 50% of the population with a confidence of 95% and a statistical power of 80%. The protocol of the study was approved by the competent research ethics committee before its initiation.
Variables and definitionsWe collected data on demographic, prenatal and obstetric characteristics, resuscitation in the delivery room and respiratory support in the first 2 weeks post birth (days 1, 3, 7 and 14), and the main outcomes associated with prematurity. The primary outcomes were BPD and moderate-severe BPD. We defined BPD as the need of supplemental oxygen at 28 days post birth (based on the nationwide SEN1500 database in Spain) and classified its severity based on oxygen requirements and need of respiratory support at 36 weeks: mild if the infant no longer required oxygen, moderate if the infant required a fraction of inspired oxygen (FiO2) of less than 30% and severe if the infant required a FiO2 of 30% or greater or invasive mechanical ventilation (IMV).17 We defined necrotising enterocolitis (NEC) as meeting the criteria for Bell stage 2 or higher.18 Patent ductus arteriosus (PDA) was diagnosed by means of echocardiography, ordered based on the judgment of the clinician, and treated per the unit protocol. We defined intraventricular haemorrhage (IVH) based on the grading system proposed by Volpe.19 All infants underwent screening for retinopathy or prematurity (ROP), which was graded based on Spanish guidelines.20 We defined intrauterine growth restriction (IUGR) as a birth weight z-score of less than –1.5 based on the Fenton growth charts.21 The diagnosis of nosocomial sepsis was based on a positive result of blood culture. Metabolic bone disease of prematurity was diagnosed based on the serum levels of inorganic phosphate and alkaline phosphatase on week 3 post birth.22
Respiratory management protocolThere were no relevant changes in respiratory management during the period under study. Resuscitation in the delivery room adhered to the recommendations of the working group on resuscitation of the Sociedad Española de Neonatología (Spanish Society of Neonatology), with initiation of respiratory support with non-invasive ventilation (NIV) with continuous positive airway pressure/non-invasive positive pressure ventilation (CPAP/NIPPV) with a T-piece resuscitator and mask.23,24 In the NICU, NIPPV was used for early rescue before contemplating orotracheal intubation (OTI) in the case of unfavourable progression of hyaline membrane disease. Surfactant was administered in the first hours post birth if oxygen requirements in NIV exceeded 30% or the patient required OTI. The method of choice for surfactant delivery in infants that were not intubated was the INtubation-SURfactant-Extubation (INSURE) procedure, during which NIV was maintained, avoiding the use of intermittent positive pressure breathing through the endotracheal tube, and following premedication with caffeine, fentanyl and atropine. Caffeine was administered for prophylaxis to all neonates born before 28 weeks of gestation and neonates delivered before 32 weeks if they developed apnoea of prematurity. Administration of a 22-day course of hydrocortisone (total dose 72.5 mg/kg) was contemplated in the case of intubated infants requiring a FiO2 greater than 30% in week 3 post birth.25 Two patients received experimental intravenous mesenchymal stem cell therapy in the framework of a clinical trial.
Statistical analysisWe performed a descriptive analysis, summarising quantitative variables as mean ± standard deviation and qualitative variables as absolute frequency and percentage distributions. We used the applicable tests in the univariate analysis: Student t or Mann–Whitney U test for quantitative data and χ2 or Fisher exact test for qualitative data. We considered p-values of less than 005 statistically significant. We performed a multivariate analysis by means of logistic to identify risk factors for BPD. In this analysis, we included variables relating to the period before the diagnosis of BPD that were statistically significant in the univariate analysis as well as variables that were not statistically significant but that we considered clinically relevant. We excluded variables that were highly correlated to one another. We generated receiver operating characteristic (ROC) curves with calculation of the area under the curve (AUC) for the final models and applied 2 predictive models for BPD previously described in the literature to our study sample26,27 for comparison. All the statistical analyses were made with the software SPSS version 24 (IBM Corp, Armonk, NY, USA).
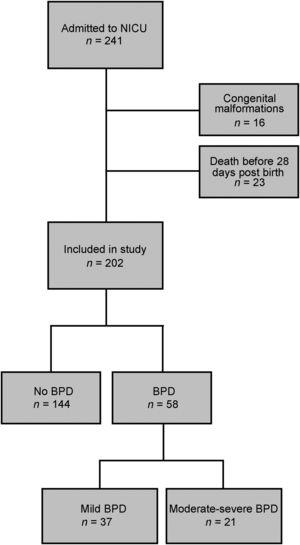
ResultsIn the period under study, a total of 241 neonates born preterm at or before 32 weeks of gestation with a birthweight of 1500 g or less. We excluded those with major congenital malformations (n = 16) and those deceased before 28 days post birth, in who it was not possible to diagnose BPD (n = 23, of who 8 died from respiratory causes). The final sample included 202 very preterm infants (106 female, 52.5%) (Fig. 1). The mean gestational age was 29.5 ± 2.1 weeks and the mean birthweight 1142.1 ± 255.5 g. Table 1 presents the main perinatal characteristics of the sample and the most relevant neonatal diagnosis and outcomes.
Descriptive analysis of study sample.
| Perinatal characteristics, mean ± SD | Total sample n = 202 | GA ≤ 28 wk, n = 77 | GA 29−32 wk, n = 125 |
|---|---|---|---|
| Gestational age (weeks) | 29.50 ± 2.1 | 27.3 ± 1.2 | 30.8 ± 1.1 |
| Birthweight (g) | 1142.08 ± 255.5 | 956.52 ± 218.5 | 1250.84 ± 212.8 |
| Weight z-score | −0.46 ± 0.8 | −0.01 ± 0.8 | −0.74 ± 0.6 |
| Maternal age (years) | 34.19 ± 6.3 | 33.8 ± 6.6 | 34.4 ± 6.2 |
| n (%) | |||
| Maternal HTN | 46 (22.8%) | 13 (16.9%) | 33 (26.4%) |
| Chorioamnionitis | 32 (15.8%) | 21 (27.3%) | 11 (8.8%) |
| Antenatal steroidsa | 198 (98%) | 77 (100%) | 121 (96.8%) |
| Multiple birth | 77 (38.1%) | 22 (28.6%) | 55 (44%) |
| Vaginal delivery | 46 (22.8%) | 20 (26%) | 26 (20.8%) |
| IVF | 51 (25.2%) | 15 (19.5%) | 36 (28.8%) |
| Sex (female) | 106 (52.5%) | 44 (57.1%) | 62 (49.6%) |
| IUGR | 18 (8.9%) | 3 (3.9%) | 15 (12%) |
| Neonatal outcomes | |||
| mean ± SD | |||
| Length of stay, NICU | 26.39 ± 18.9 | 41.29 ± 19.1 | 17.2 ± 11.5 |
| Length of stay, total | 61.14 ± 55.6 | 85.8 ± 82.5 | 45.83 ± 14.2 |
| n (%) | |||
| BPD | 58 (28.7%) | 50 (64.9%) | 8 (6.4%) |
| moderate-severe BPD | 21 (10.4%) | 17 (22.1%) | 4 (3.2%) |
| Oxygen at discharge | 8 (4%) | 6 (7.8%) | 2 (1.6%) |
| PDA | 41 (20.3%) | 31 (40.3%) | 10 (8%) |
| Pharmacological closure of PDA | 29 (14.4%) | 24 (31.2%) | 5 (4%) |
| Surgical closure of PDA | 12 (5.9%) | 9 (11.7%) | 3 (2.4%) |
| NEC | 11 (5.4%) | 7 (9.1%) | 4 (3.2%) |
| ROP grade > II | 22 (10.9%) | 20 (26.3%) | 14 (11.2%) |
| Nosocomial sepsis | 73 (36.1%) | 41 (53.2%) | 32 (25.6%) |
| IVH grade > II | 12 (5.9%) | 11 (14.3%) | 1 (0.8%) |
| PVL | 13 (6.4%) | 10 (13%) | 3 (2.4%) |
| MBD | 42 (20.8%) | 21 (27.3%) | 21 (16.8%) |
AUC, area under the curve; BPD, bronchopulmonary dysplasia; GA, gestational age; HTN, hypertension; IQR, interquartile range; IUGR, intrauterine growth restriction; IVF, in vitro fertilization; IVH, intraventricular haemorrhage; MBD, metabolic bone disease; NEC, necrotising enterocolitis; NICU, neonatal intensive care unit; PDA, patent ductus arteriosus; PVL, periventricular leukomalacia; ROP, retinopathy of prematurity; SD, standard deviation; wk, week.
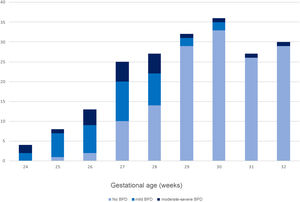
A total of 58 patients (28.7%) received a diagnosis of BPD, of who 21 (10.4% of the total) were classified as cases of moderate-severe BPD; Fig. 2 presents the distribution of cases by weeks of gestational age. Forty neonates (19.8%) required intubation in the delivery room, and 101 (50%) required surfactant during the stay. Seventy-eight (38.6%) received IMV during the stay, and the rest of the patients (n = 124) only required non-invasive respiratory support. None of the patients died after 28 days post birth.
Table 2 summarises the results of the univariate analysis for BPD. The most relevant independent risk factors for BDP identified in the logistic regression analysis were gestational age (odds ratio [OR], 0.44; 95% CI = 0.30−0.65; P < 0.001), the need of IMV in the first day post birth (OR, 8.13; 95% CI = 2.41–27.42; P = 0.001), nosocomial sepsis (OR, 9.51; 95% CI = 2.99–30.28; P < 0.001) and the FiO2 on day 14 post birth (OR, 1.39; 95% CI = 1.16–1.66; P < 0.001) (Fig. 3). The AUC for the BPD model was 0.967 (95% CI, 0.944–0.990).
Risk factors for bronchopulmonary dysplasia.
| BPD (n = 58) | No BPD (n = 144) | p | AUC | 95% CI | |
|---|---|---|---|---|---|
| Perinatal characteristics | |||||
| mean ± SD | |||||
| Gestational agea | 27.5 ± 1.7 | 30.3 ± 1.6 | <0.001 | 0.888 | 0.77−1 |
| Weight (g)b | 938.7 ± 216.8 | 1224 ± 222.5 | <0.001 | 0.790 | 0.62−0.96 |
| Weight z-scoreb | −0.2 ± 0.9 | −0.6 ± 0.8 | 0.005 | 0.628 | 0.54−0.72 |
| Maternal age (years) | 34.2 ± 6.9 | 34.2 ± 6.1 | 0.980 | 0.500 | 0.41−0.59 |
| n (%) | RR | 95% CI | |||
| Maternal hypertension | 12 (20.7%) | 34 (23.6%) | 0.654 | 0.885 | 0.51−1.52 |
| Chorioamnionitis | 10 (17.2%) | 22 (15.3%) | 0.729 | 1107 | 0.63−1.95 |
| Antenatal steroidsc | 57 (98.3%) | 141 (97.9%) | 0.868 | 1515 | 0.21−6.37 |
| Multiple birth | 19 (32.8%) | 58 (40.3%) | 0.319 | 0.791 | 0.49−1.36 |
| IVF | 12 (20.7%) | 39 (27.1%) | 0.344 | 0.772 | 0.44−1.34 |
| Vaginal delivery | 17 (29.3%) | 29 (20.1%) | 0.160 | 1406 | 0.89−2.23 |
| Female sex | 27 (46.6%) | 79 (54.8%) | 0.285 | 0.789 | 0.51−1.22 |
| IUGR | 3 (5.2%) | 15 (10.4%) | 0.237 | 0.557 | 0.19−1.60 |
| Resuscitation in delivery room | |||||
| n (%) | RR | 95% CI | |||
| Oxygenb | 55 (94.8%) | 114 (79.2%) | 0.006 | 3580 | 1.29−10.76 |
| OTIb | 24 (41.4%) | 16 (11.1%) | <0.001 | 2859 | 1.93−4.23 |
| Cardiac massage | 4 (6.9%) | 6 (4.2%) | 0.418 | 1422 | 0.64−3.14 |
| Adrenaline | 3 (5.2%) | 4 (2.8%) | 0.400 | 1519 | 0.63−3.68 |
| Neonatal outcomes | |||||
| mean ± SD | AUC | 95% CI | |||
| FiO2 day 1b | 28.9 ± 13.1 | 23.9 ± 5.5 | 0.007 | 0.661 | 0.58−0.74 |
| FiO2 day 3b | 26 ± 7.5 | 22.3 ± 3.1 | 0.001 | 0.699 | 0.61−0.78 |
| FiO2 day 7b | 25.4 ± 6.2 | 21.7 ± 2.4 | <0.001 | 0.709 | 0.62−0.80 |
| FiO2 day 14a | 27.8 ± 7.2 | 21.4 ± 1.9 | <0.001 | 0.835 | 0.76−0.91 |
| n (%) | RR | 95% CI | |||
| Surfactantb | 50 (86.2%) | 51 (35.4%) | <0.001 | 6250 | 3.12−12.50 |
| nCPAP | 58 (100%) | 137 (95.1%) | 0.087 | – | – |
| NIPPVb | 57 (98.3%) | 53 (36.8%) | <0.001 | 47,673 | 6.73−337.63 |
| CMVb | 50 (86.2%) | 28 (19.4%) | <0.001 | 9936 | 1.98−19.82 |
| HFOVb | 5 (8.6%) | 3 (2.1%) | 0.031 | 2288 | 1.28−4.10 |
| IMV day 1 post birtha | 36 (62.1%) | 15 (10.4%) | <0.001 | 4845 | 3.17−7.41 |
| IMV day 3 post birthb | 18 (31%) | 3 (2.1%) | <0.001 | 3878 | 2.80−5.36 |
| IMV day 7 post birthb | 12 (20.7%) | 1 (0.7%) | <0.001 | 3792 | 2.82−5.10 |
| IMV day 14 post birthb | 17 (29.3%) | 1 (0.7%) | <0.001 | 4238 | 3.16−5.68 |
| PDAb | 26 (44.8%) | 15 (10.4%) | <0.001 | 3190 | 2.16−4.70 |
| Pharmacological closure of PDAb | 20 (34.5%) | 9 (6.3%) | <0.001 | 3140 | 2.16−4.55 |
| Surgical closure of PDAb | 9 (15.5%) | 3 (2.1%) | <0.001 | 2908 | 1.94−4.36 |
| Sepsis nosocomiala | 41 (70.7%) | 32 (22.2%) | <0.001 | 4262 | 2.62−6.94 |
AUC, area under the curve; CI, confidence interval; CMV, conventional mechanical ventilation; HFOV, high-frequency oscillatory ventilation; HTN, hypertension; IMV, invasive mechanical ventilation; IUGR, intrauterine growth restriction; IVF, in vitro fertilization; nCPAP, nasal continuous positive airway pressure; NIPPV, non-invasive positive pressure ventilation; OTI, orotracheal intubation; PDA, patent ductus arteriosus; RR, relative risk; SD, standard deviation.
Table 3 summarises the results of the univariate analysis for moderate-to-severe BPD. The most relevant independent risk factors for moderate-severe BDP identified in the logistic regression analysis were the need of IMV on days 1 (OR, 5.39; 95% CI = 1.54–18.89; P = 0.008) and 3 of life (OR, 9.99; 95% CI = 2.47–40.44; P = 0.001) and diagnosis of nosocomial sepsis (OR, 9.87; 95% CI = 2.58–37.80; P = 0.001) (Fig. 3). The AUC for the moderate-severe BPD model was 0.891 (95% CI, 0.812–0.971).
Risk factors for moderate-to-severe bronchopulmonary dysplasia.
| Moderate-severe BPD (n = 21) | No moderate-severe BPD (n = 181) | p | AUC | 95% CI | |
|---|---|---|---|---|---|
| Perinatal characteristics | |||||
| mean ± SD | |||||
| Gestational ageb | 27.78 ± 2 | 29.7 ± 2 | <0.001 | 0.447 | 0.12−0.79 |
| Weight (g)b | 923.1 ± 268.3 | 1167.5 ± 242.2 | <0.001 | 0.404 | 0.10−0.70 |
| Weight z-score | −0.5 ± 0.8 | −0.4 ± 0.8 | 0.604 | 0.649 | 0.30−0.99 |
| Maternal age (years) | 33 ± 6.3 | 34.3 ± 6.3 | 0.365 | 0.429 | 0.30−0.56 |
| n (%) | RR | 95% CI | |||
| Maternal HTN | 6 (28.6%) | 40 (22.1%) | 0.503 | 1356 | 0.56−3.30 |
| Chorioamnionitis | 2 (9.5%) | 30 (16.6%) | 0.402 | 0.559 | 0.14−2.28 |
| Antenatal steroidsa | 20 (95.2%) | 178 (98.3%) | 0.334 | 0.404 | 0.07−2.32 |
| Multiple birth | 9 (42.9%) | 68 (37.6%) | 0.637 | 1217 | 0.54−2.75 |
| IVF | 4 (19%) | 47 (26%) | 0.490 | 0.696 | 0.25−1.97 |
| Vaginal delivery | 6 (28.6%) | 40 (22.1%) | 0.552 | 1356 | 0.56−3.30 |
| Sex (female) | 8 (38.1%) | 98 (54.1%) | 0.163 | 0.557 | 0.24−1.29 |
| IUGR | 1 (4.8%) | 17 (9.4%) | 0.481 | 0.511 | 0.07−3.60 |
| Resuscitation in delivery room | |||||
| n (%) | RR | 95% CI | |||
| Oxygenb | 21 (100%) | 148 (81.8%) | 0.032 | – | – |
| OTIc | 8 (38.1%) | 32 (17.7%) | 0.026 | 2492 | 1.11−5.60 |
| Cardiac massage | 1 (4.8%) | 9 (5%) | 0.966 | 0.960 | 0.14−6.45 |
| Adrenaline | 0 | 7 (3.9%) | 0.359 | – | – |
| Neonatal outcomes | |||||
| mean ± SD | AUC | 95% CI | |||
| FiO2 day 1b | 32.9 ± 17.2 | 24.5 ± 6.6 | 0.007 | 0.733 | 0.61−0.85 |
| FiO2 day 3b | 27.7 ± 8.2 | 22.9 ± 4.3 | 0.037 | 0.716 | 0.59−0.85 |
| FiO2 day 7b | 26.6 ± 6.6 | 22.3 ± 3.7 | 0.015 | 0.709 | 0.57−0.84 |
| FiO2 day 14b | 29.7 ± 8.1 | 22.5 ± 4 | 0.009 | 0.826 | 0.72−0.93 |
| n (%) | RR | 95% CI | |||
| Surfactantb | 20 (95.2%) | 81 (44.8%) | <0.001 | 20,000 | 2.74−146.21 |
| nCPAP | 21 (100%) | 174 (96.1%) | 0.359 | – | – |
| NIPPVb | 21 (100%) | 89 (49.2%) | 0.010 | – | – |
| CMVb | 20 (95.2%) | 58 (32%) | <0.001 | 31,795 | 4.35−232.20 |
| HFOVb | 3 (14.3%) | 5 (2.8%) | 0.010 | 4042 | 1.49−10.95 |
| IMV day 1 post birthc | 16 (76.2%) | 36 (19.9%) | <0.001 | 9474 | 3.65−24.56 |
| IMV day 3 post birthc | 11 (52.4%) | 10 (5.5%) | <0.001 | 9481 | 4.58−19.62 |
| IMV day 7 post birthb | 6 (28.6%) | 7 (3.9%) | <0.001 | 5815 | 2.71−12.46 |
| IMV day 14 post birthb | 8 (38.1%) | 10 (5.5%) | <0.001 | 6290 | 3.01−13.13 |
| PDAb | 10 (47.6%) | 31 (17.1%) | 0.001 | 3570 | 1.63−7.82 |
| Pharmacological closure of PDAb | 7 (33.3%) | 22 (12.2%) | 0.009 | 2983 | 1.32−6.76 |
| Surgical closure of PDAb | 5 (23.8%) | 7 (3.9%) | <0.001 | 4948 | 2.18−11.20 |
| Nosocomial sepsisc | 17 (81%) | 56 (30.9%) | <0.001 | 7510 | 2.63−21.48 |
AUC, area under the curve; CI, confidence interval; CMV, conventional mechanical ventilation; HFOV, high-frequency oscillatory ventilation; HTN, hypertension; IMV, invasive mechanical ventilation; IUGR, intrauterine growth restriction; IVF, in vitro fertilization; nCPAP, nasal continuous positive airway pressure; NIPPV, non-invasive positive pressure ventilation; OTI, orotracheal intubation; PDA, patent ductus arteriosus; RR, relative risk; SD, standard deviation.
The AUCs for the ROC curves of the previously published models selected for comparison were smaller compared to the AUCs obtained in our models. In the case of Gursoy et al.,27 the model for prediction of BPD had an AUC of 0.869 (95% CI, 0.815−0.922) and the model for moderate-severe BPD an AUC of 0.803 (95% CI, 0.711−0.895). In the case of Hunt et al.,26 the model for prediction of BPD had an AUC of 0.600 (95% CI, 0.508−0.692) and the model for moderate-severe BPD an AUC of 0.624 (95% CI, 0.480−0.767).
DiscussionA better understanding of the risk factors for BPD is a key step toward the prevention and adequate management of this disease. Our study, conducted in a consecutive sample of infants delivered at or before 32 weeks of gestation with birthweights of 1500 g or less, most of who were managed with non-invasive support, and with an incidence of BPD similar to the incidence described in the previous literature, corroborated that lesser GA, the need of IMV in the first days post birth and nosocomial infection are the main early risk factors for BPD. The predictive ability of our models improved compared to past models.26,27
In our patients, the incidence of moderate-severe BPD was 10.4%, lower compared to the national mean (15.8%) based on data from the SEN1500 study published by the iNeo network,11 which reported a significantly higher global incidence for participating countries (25.5%). These improved outcomes were also reflected in the survival free of moderate-severe BPD in infants delivered at or before 28 weeks, which was of 79.2% in our sample, greater than the national mean (61.4%), but similar to survival in hospitals in our region that have reported more favourable outcomes (72.5%).28 A factor that may play a role in these results is the lower frequency of extremely preterm infants in our case series.
It is widely accepted that gestational age is the best isolated predictor of BPD,7 although in most instances this is not a modifiable factor. At birth, the respiratory prognosis is often influenced by the cause of preterm birth and by the resulting interruption of foetal lung maturation.7,9 Thus, gestational birth is a very important factor in the most premature infants, who are born at a very early stage of lung maturation.7 It is highly probable that these infants will develop BPD independently of how they are managed after birth.9 Notwithstanding, there are multiple postnatal factors that can play a role in the severity of BPD and therefore influence its outcomes, especially in less preterm infants. The activation of the inflammatory cascade and oxidative stress are 2 of the key pathophysiological pathways leading to the development of BPD. In association with this physiological basis, factors such as positive airway pressure, supplemental oxygen and postnatal sepsis have also been found to be related to BPD,7 as occurred in our study.
Several studies have described patterns of disease that can usually be identified in the first weeks of life.29,30 First, there are infants with mild pulmonary disease at birth that recover gradually. Then, there are infants that experience early persistent pulmonary deterioration and usually require prolonged respiratory support starting from birth. Lastly, there are infants who recover from initial lung disease but later experience respiratory decompensation.7 This goes beyond what Northway described in 1967 in patients with BPD, then defined as the pulmonary disease developed by preterm infants subjected to prolonged mechanical ventilation.31 Today’s preterm infants do not have the same characteristics and are managed differently compared to the population in which BPD was first defined. Strategies used to improve lung development outcomes (used of synchronised ventilation modalities, standardization of lung volumes, increased use of NIV, early administration of surfactant and antenatal steroids) have resulted in the different population of patients we manage today.29 Every infant in our study was managed with these methods, as we obtained a consecutive sample of infants managed in the past decade. Thus, fewer than 4 in 10 patients received IMV during the hospital stay, and fewer than 2 in 10 required endotracheal intubation at birth. Notwithstanding, there is still a group of patients in whom the need of intubation and of IMV cannot be avoided.
When we analysed the relationship between IMV and BPD in the sample, we found that the need of IMV in the first days post birth was independently associated with the development of BPD, as previously described.32,33 Sixty percent of the patients that required intubation during stabilization in the delivery room developed BPD, and the percentage increased to 70% in those who required IMV in the first day of life. In contrast, only 14% of patients that were not intubated in the first day of life developed BPD. Similarly, nearly 9 out of 10 patients that needed IMV on day 3 post birth developed BPD and more than 50% moderate-severe BPD.
Our findings evinced another opportunity for improvement in the use of NIV for initial respiratory support. In our sample of very and extremely preterm infants, 8 in 10 received non-invasive respiratory support in the delivery room. In the group of patients that developed BPD, 60% had received CPAP/NIPPV at birth. However, this proportion decreased to 40% in the first 24 h post birth. Thus, there was a sizeable group of patients in who NIV failed in the first hours post birth and who eventually developed BPD. This was consistent with the findings in other case series in which CPAP failure was associated with an increase in morbidity, including BPD.34 Thorough investigation of these cases to establish NIV protocols and improve the respiratory management of these patients is an essential step that could help avoid IMV in a particularly important stage of disease development.33,35,36
Multiple previous studies have demonstrated the association between postnatal sepsis and the development of pulmonary inflammation.37–39 Some have found evidence suggestive of a causal relationship between postnatal infection, PDA and increased duration of IMV.37,39 All these factors are probably interrelated. However, in our study nosocomial sepsis remained an independent risk factor in the final logistic regression model. In fact, its presence was associated with a more than 9-fold increase in the risk of BPD as well as with the severity of BPD. Thus, in our population, nosocomial sepsis should be considered a risk factor in and of its own. Improving the rate of nosocomial infection through stricter protocols and limited use of invasive procedures is another of the preventive strategies that should be prioritised.
There are limitations to our study, including its retrospective design, performance in a single centre and small sample size, in addition to the inclusion of preterm infants born between 28 and 32 weeks of gestation, in who the baseline risk of BPD is low. The definition of BPD as oxygen dependence at 28 days post birth also carries intrinsic limitations, such as the discrepancy with the criterion established in the 2001 consensus (dependency for 28 days). This could hinder comparisons with the results of other studies. The values of the AUCs in the ROC curves of our models should be interpreted with caution, as we did not select variables for application at specific time points and we did not correct for the overestimation that may result from testing the model in the same patients from who it was derived. Our conclusions should be analysed with prudence and are only directly applicable to the population under study. On the other hand, the study was conducted in a homogeneous consecutive sample spanning 7 years during which the same protocols were applied and there were no significant changes in clinical management, which was akin to clinical practice in other facilities in our region.
In conclusion, the analysis of simple and objective data available in the first days post birth, such as GA at birth and the need of IMV in days 1 and 3 of life, can help identify a subset of patients at high risk of BPD in whom more aggressive management may be warranted. Our findings may provide the foundation to develop early-stage predictive models or to compare the predictive power of new tools using clinical data that can be obtained at the bedside.
Data availabilityData will be made available on request.
FundingThis research did not receive any external funding.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Sucasas Alonso A, Pértega Díaz S, Sáez Soto R, Avila-Alvarez A. Epidemiología y factores de riesgo asociados a displasia broncopulmonar en prematuros menores de 32 semanas de edad gestacional. An Pediatría. 2022;96:242–251.