To estimate the impact of the incorporation of high-flow nasal cannule (HFNC) in patients admitted with acute bronchiolitis in a hospital without pediatric intensive care unit (PICU).
Material and methodsCohort study with historical control of bronchiolitis in a second-level hospital, before (2009−2012) and after (2015−2020) the implementation of HFNC. The main outcome was the need for admission to the PICU.
Results301 patients were included. Respiratory syncytial viruses were identified in 64.7% of them and influenza viruses in 0.3%. No differences in age nor comorbility between periods were observed. The average stay was 3.67 days (standard deviation [SE] 2.10) in the first period and 4.00 days (SE 2.35) in the second. Three patients were transferred to UCIP (2.6%) before the availability of HFCN and 13 patients (9.4%) after, which supposed an important increase of the risk (relative risk 3.58; confidence interval [CI] 95%: 1.04–12.27), although not significant in adjusted analyses (Odds ratio 3.48; IC95% 0.95–12.72). A significant increase in readmission risk was also observed (from 5.3%–13.7%) and a shortening of the time to transfer.
ConclusionsThe incorporation of HFNC was not associated with a lower risk of transfer to PICU nor a shorter length of oxygen therapy. In the absence of evidence, that supports the effectiveness and efficiency of the HFNC and establishes its indications, we must reassess its use.
Evaluar el impacto de la incorporación de la oxigenoterapia de alto flujo (OAF) en pacientes ingresados con bronquiolitis aguda en un hospital sin unidad de cuidados intensivos pediátricos (UCIP).
Material y métodosEstudio de cohortes con control histórico de bronquiolitis ingresadas en un hospital de segundo nivel, antes (2009−2012) y después (2015−2020) de la introducción de OAF. La medida principal de efecto fue necesidad de traslado a UCIP.
ResultadosSe incluyeron 301 pacientes. En el 64,7% se identificó virus respiratorio sincitial y en el 0,3% virus de la gripe. No se observaron diferencias en la edad ni en la comorbilidad entre períodos. La media de la estancia en planta fue de 3,67 días (desviación estándar [DE] 2,10) en la primera etapa y de 4,00 días (DE 2,35) en la segunda etapa. Fueron trasladados a UCIP tres pacientes en el período 2009−2012 (2,6%) y 13 pacientes (9,4%) en el período 2015−2020 lo que supuso un importante aumento de riesgo (riesgo relativo 3,58; intervalo de confianza [IC] 95%: 1,04 a 12,27), aunque no significativo en los análisis ajustados (Odds ratio 3,48; IC95% 0,95 a 12,72).
También se observó un aumento significativo de reingresos (de 5,3% a 13,7%) y un acortamiento del tiempo hasta traslado.
ConclusionesLa incorporación de la OAF en planta no se asoció a menor riesgo de traslado a UCIP ni menor duración de la oxigenoterapia. En ausencia de evidencia que apoye la eficacia y eficiencia de la OAF y establezca sus indicaciones, debemos revaluar su uso.
Respiratory disease is currently the leading cause of hospital admission during childhood. Within this category, bronchiolitis is one of the most prevalent diseases, accounting for the bulk of admissions during the winter, and increasing the health care burden during those months due to the need to provide respiratory support to affected patients (approximately 1 in 3 babies will develop clinical bronchiolitis in the first year of life and 2%–3% of all babies will need to be admitted to hospital, based on data from National Institute for Health and Care Excellence [NICE] of the Department of Health and Social Care of the United Kingdom1). Between 3% and 11% of the patients require admission to the intensive care unit.2,3 Thus, despite its low mortality, bronchiolitis is a disease that leads to extensive use of health care resources in the epidemic season.4,5
Despite its frequency and the high use of health care resources, there is still considerable controversy as regards its optimal management.6 The appropriate pharmacological treatment remains to be established, as most of the treatments that have been tried have proven innefective7 in evidence of high quality.8,9
At present, medical management is based on general supportive care, including respiratory care, delivered in a broad range of modalities that may include low-flow oxygen therapy delivered with nasal prongs, non-invasive ventilation (NIV), conventional mechanical ventilation (CMV) or high-flow oxygen therapy (HFOT), which was introduced in the last decade.
High-flow oxygen therapy consists in the delivery of oxygen, pure or mixed with air, at a rate exceeding the air requirements of the patient (flow rates of 1−8 L/min in infants or 5−40 L/min in adults). It is provided with devices designed specifically for the purpose such as the Fisher & Paykel®, Vapotherm® or WILAmed® high-flow nasal cannulae (HFNC), available in different sizes depending on the desired flow rate, which deliver a mix of air and oxygen (at a concentration nearing 100%) that is humidified and heated (to 34−37 °C). The evidence on its efficacy, efficiency and specific indications for the management of acute bronchiolitis is still insufficient. However, its use is becoming widespread in both emergency departments and inpatient wards.7
The aim of our study was to describe the differences in the frequency of transfers to the paediatric intensive care unit (PICU), days of oxygen therapy, frequency of readmission and pharmaceuticals used in patients with acute bronchiolitis admitted to a secondary care hospital in 2 time periods, one in which HFOT was not available and one in which it was.
Material and methodsWe conducted a cohort study with historical controls including patients aged less than 24 months with a diagnosis of acute bronchiolitis that required admission to the paediatric ward of a general hospital that does not have a PICU between January 2009 and February 2020. We compared cases in a period before HFOT was available (2009−2012) and a period when HFOT was already available (2015−2020), considering 2013−2014 a transitional period that was therefore excluded from the analysis (in these 2 years, the equipment needed to provide HFOT was not available for every admitted patient, nor was all the staff trained in its use).
We searched the electronic health records database of the unit to identify every patient admitted with this disease in the periods under study. We collected data through the review of the health records. The study was approved by the ethics committee for research with medicinal products of the health district where the hospital was located.
We excluded patients that, despite having received a diagnosis of bronchiolitis, did not meet the McConnochie criteria for definition of bronchiolitis,10 most frequently because the episode was not the first episode of wheezing experienced by the patient, or the patient was aged more than 24 months.
In January 2015, HFOT was introduced in the paediatric ward as a respiratory support modality, initially with the MR850 Fisher&Paykel® system and later on with the AIRVO2 Fisher&Paykel® system, and in the last winter, there were 3 devices available in the ward.
This modality of respiratory support started to be used in the department of paediatrics in 2015 following the recommendation of different paediatrics societies and after delivery of basic training on its correct use to the medical and nursing staff. The patients that received HFOT were monitored with conventional pulse oximetry during its delivery. High-flow oxygen therapy was initiated in patients after being assessed by the paediatrician in charge of the inpatient ward at the time of admission and/or if the symptoms worsened.
We collected data on clinical and epidemiological variables: age, sex, date of birth, relevant personal history (preterm birth, chronic pulmonary, cardiac or neuromuscular disease, polymalformative genetic syndromes, previous infection), collection of nasopharyngeal aspirate sample for detection of respiratory syncytial virus (RSV) and influenza, date of admission, date of discharge, days of HFOT, days of oxygen therapy, transfer to PICU, blood gas analysis, Wood-Downes and Hospital Sant Joan de Déu clinical scoring systems, pharmacological treatment (adrenaline, salbutamol, ipratropium bromide, hypertonic saline, steroid therapy, antibiotherapy) and need of readmission. We defined readmission as need for admission to the unit within 15 days from discharge due to a new episode of respiratory distress.
We have described qualitative variables as absolute and relative frequency distributions of their categories. We summarised continuous data as mean and standard deviation (SD) if they fit a normal distribution, and otherwise as median and interquartile range (IQR). We calculated risk statistics and mean differences between periods with the corresponding 95% confidence intervals (CIs). We compared qualitative variables in different periods by means of the chi square test or exact test and quantitative variables by means of the Student t test or Mann-Whitney U test. We calculated the statistical power a posteriori for pre-intervention non-inferiority testing for the risk of transfer to the PICU, with a lower limit for the difference of proportions between periods of −1%, estimating a power of 83% (alpha level, 5%; inverse of the observed difference, 6.8%). We conducted an unconditional logistic regression analysis to estimate the risk of transfer to the PICU adjusted for age, sex, detection of de RSV, severity, use of steroid therapy, salbutamol, adrenaline and hypertonic saline; we used a backward stepwise approach basing the removal of variables on differences in the likelihood ratio. We also conducted a survival analysis of the time to transfer with calculation of adjusted hazard ratios by means of Cox regression (censoring cases that did not require transfer before discharge).
ResultsWe included a total of 301 with a diagnosis of bronchiolitis in the 11 years under study. Table 1 presents the distribution and characteristics of the patients by period. We found that 31.7% of patients received respiratory support with HFOT at some point during the hospital stay.
Characteristics of patients with bronchiolitis by period.
| Period | 2009−2012 | 2013−2014 | 2015−2020 | P* |
|---|---|---|---|---|
| Number admitted | 115 | 47 | 139 | |
| Age (months): mean ± SD | 5.20 ± 4.78 | 4.61 ± 4.04 | 4.96 ± 4.88 | |
| median (IQR) | 3.51 (1.74−7.29) | 3.25 (1.64−7.72) | 3.02 (1.38−6.57) | .341 |
| Age <3 months (%) | 47 (40.9%) | 23 (48.9%) | 69 (49.6%) | .347 |
| Male sex (%) | 68 (59.1%) | 32 (68.1%) | 78 (56.1%) | .628 |
| Respiratory syncytial virus (%) | 72 (63.2%) | 33 (70.2%) | 89 (64.0%) | .886 |
| Influenza (%) | 0 (0.0%) | 0 (0.0%) | 1 (0.7%) | 1.000 |
| Comorbidity (%) | 40 (34.8%) | 16 (34.0%) | 33 (23.7%) | .053 |
| Blood gas analysis (%) | 60 (52.6%) | 27 (57.4%) | 64 (46.4%) | .323 |
| Steroid therapy (%) | 52 (45.6%) | 26 (55.3%) | 34 (24.5%) | <.001 |
| Salbutamol (%) | 100 (87.7%) | 42 (89.4%) | 104 (74.8%) | .010 |
| Adrenaline (%) | 25 (21.9%) | 8 (17.0%) | 41 (29.5%) | .173 |
| Hypertonic saline (%) | 68 (59.6%) | 7 (14.9) | 43 (30.9%) | <.001 |
| Antibiotherapy (%) | 28 (24.6%) | 9 (19.1%) | 27 (19.4%) | .324 |
| Readmission (%) | 6 (5.3%) | 7 (14.9%) | 19 (13.7%) | .026 |
| Transfer to PICU (%) | 3 (2.6%) | 3 (6.4%) | 13 (9.4%) | .028 |
| Age of patients transferred to PICU, mean ± SD | 1.20 ± 0.39 | 1.34 ± 0.40 | 0.45 ± 3.64 | |
| Median (IQR) | 1.34 (1.05−1.43) | 1.51 (1.20−1.57) | 1.28 (0.69−2.20) | .84 |
| Wood-Downes score, mean ± SD | 4 ± 1 | 4 ± 1 | 5.1 ± 1.4 | |
| Median (IQR) | 4 (3−5) | 4 (3−5) | 5 (4−6) | .083 |
| Use of HFOT (%) | 0 (0.0%) | 5 (10.6%) | 45 (32.4%) | – |
| Days of oxygen therapy, mean ± SD | 1.52 ± 2.04 | 1.72 ± 1.96 | 2.40 ± 2.42 | |
| Median (IQR) | 1 (0−2) | 1 (0−3) | 2 (0−4) | .002 |
| Days of HFOT, mean ± SD | 0.34 ± 1.22 | 0.99 ± 1.78 | – | |
| Median (IQR) | 3 (1.5−5.5) | 3 (1−4.5) | ||
| Length of stay, mean ± SD | 3.64 ± 2.11 | 3.34 ± 2.14 | 3.79 ± 2.37 | |
| Median (IQR) | 4 (2−5) | 3 (2−4) | 3 (2−5) | .793 |
| Mean days to transfer ± SD | 2.66 ± 2.88 | 1.00 ± 1.00 | 1.84 ± 1.67 | |
| Median (IQR) | 1 (1−6) | 1 (0−2) | 2 (1−3) | .038 |
| Length of stay in PICU, median (IQR) | 4 (1−7) | – | 4 (3−7) | .885 |
| Mechanical ventilation, n/N (%) | 1/3 (33.3%) | – | 2/13 (15.38%) | .473 |
HFOT, high-flow oxygen therapy; IQR, interquartile range; n/N, events/patients for who information was available; PICU, paediatric intensive care unit; SD, standard deviation.
We did not find differences between periods in patient age or sex or the proportion of cases with infection by RSV or influenza. The frequency of comorbidities was slightly lower in the 2015−2020 period, but the difference was not statistically significant
We found a decreasing trend in the use of steroid therapy, hypertonic saline and beta-2 blockers, but not in the use of adrenaline or antibiotherapy. We found no change in the performance of blood gas analysis or the proportion of patients with RSV or influenza. The mean length of stay remained stable between periods.
In the first period before the introduction of HFOT, 9 patients received respiratory support with continuous positive airway pressure (CPAP) delivered via nasal prongs with the Infant Flow Driver® system, of who 3 required transfer to the PICU; in the period when HFOT was available, CPAP was not used in any of the patients.
In the 2015−2020 period, 32.4% of patients received HFOT for a median of 1.28 days (IQR, 0.69−2.20 days). We found a greater risk of transfer to the PICU (relative risk [RR], 3.58; 95% CI, 1.04–12.27) and readmission (RR, 2.84; 95% CI, 1.17–6.90) and a longer duration of oxygen therapy (mean difference, 0.88 days; 95% CI, 0.32–1.44). There was no significant difference between periods in the mean age of transferred patients, the length of stay in the PICU of transferred patients or the need of mechanical ventilation. Supplemental Table I in Appendix B presents an analysis that incorporates the intermediate period (2013−2014) to the first period, and the comparison of this combined early period to the period with HFOT only yielded differences in the presence of comorbidities, which was more frequent in the early period, and the length of stay, differences that were not statistically significant.
Table 2 presents the analysis adjusted for age, sex, severity and presence of RSV. The only variables associated with transfer to the PICU were the period (2015−2020; P = .034) and age less than 3 months (P < .001), although for the 2015−2020 period, the confidence interval was bordering the limit of significance (odds ratio [OR], 3.48; 95% CI, 0.95–12.72). We obtained similar results when we also adjusted the analysis for the use of adrenaline, salbutamol, hypertonic saline and steroid therapy (OR, 3.37; 95% CI, 0.89–12.72); since there were significant changes between periods in these variables, the estimates were less precise.
Final multivariate logistic regression analysis model for the risk of transfer to a paediatric intensive care unit.
| Variable | Coefficient | P | OR | 95% CI | |
|---|---|---|---|---|---|
| Model 1 | |||||
| Age <3 months | 1.664 | .011 | 5.280 | 1.455 | 19.155 |
| 2015−2020 vs 2009−2012 | 1.248 | .059 | 3.485 | 0.954 | 12.724 |
| Constant | –4.612 | <.001 | |||
| Model 2 | |||||
| 2015−2020 vs 2009–2012 | 1.216 | .073 | 3.372 | 0.894 | 12.720 |
| Adrenaline | 2.656 | <.001 | 14.243 | 3.881 | 52.279 |
| Constant | –4.920 | <.001 | |||
CI, confidence interval; OR, odds ratio.
Model 1, independent variables: period, age <3 months, severe disease at admission (Wood-Downes >4), age, sex and presence of respiratory syncytial virus.
Model 2, independent variables: period, age <3 months, severe disease at admission (Wood-Downes >4), age, male sex, presence of respiratory syncytial virus, adrenaline, steroid therapy, salbutamol and hypertonic saline.
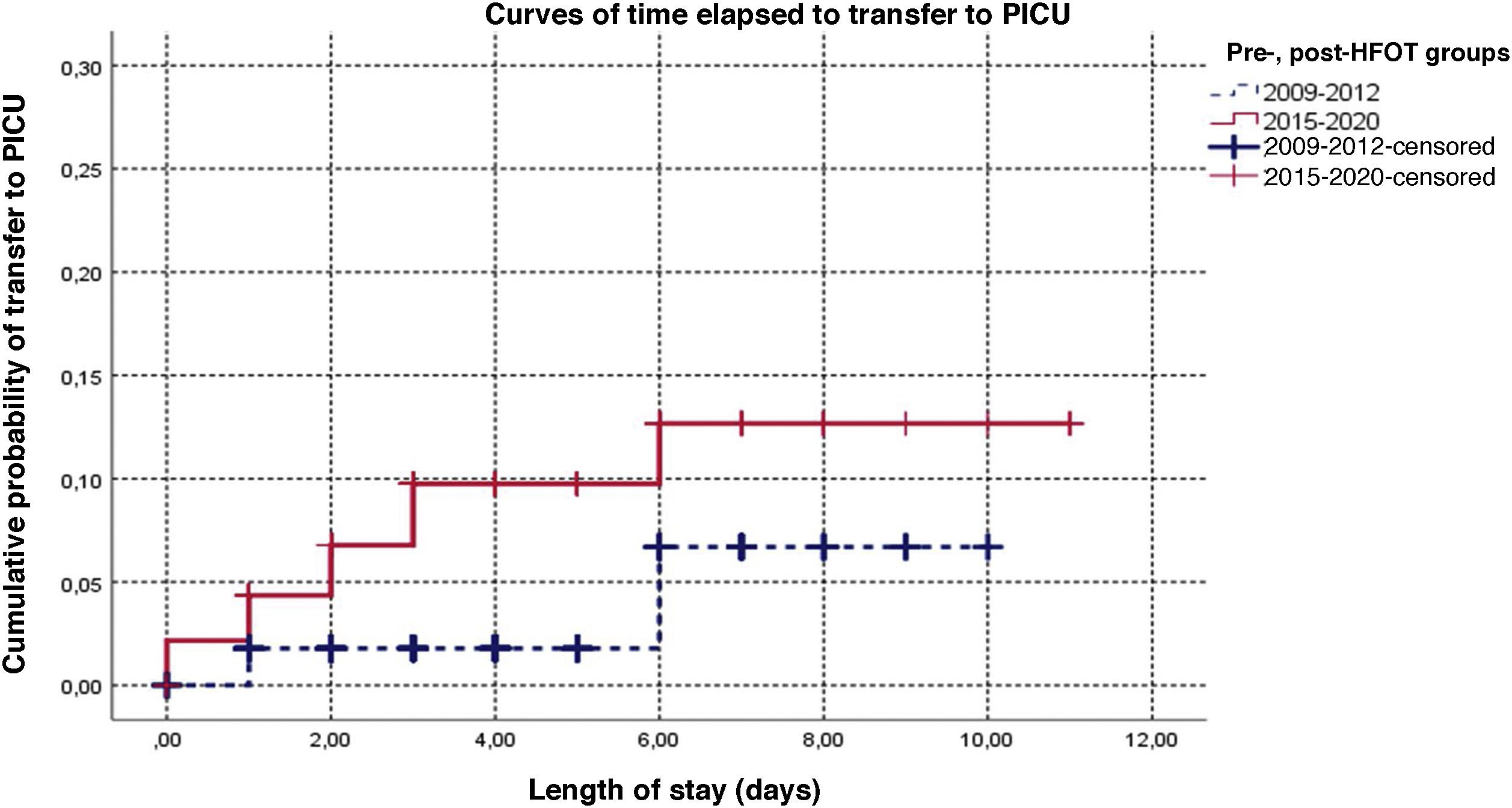
Fig. 1 presents the survival curve for the time elapsed to transfer for the periods before and after the introduction of HFOT. In the patients transferred in the latest period, transfer occurred earlier (P = .034), with an estimated 3-fold increase in risk, although the adjusted estimate was not statistically significant (HR, 3.07; 95% CI: 0.87–10.80; P = .081; Appendix B supplemental table II). Supplemental Figure I in Appendix B shows the comparison in which the early period is combined with the intermediate period (2013−2014); although the time to transfer was shorter in the 2015−2020 period, the difference was not statistically significant (P = .066).
DiscussionThe purpose of the study was to obtain a general perspective of the management of patients with bronchiolitis admitted to our paediatric ward through the years and explore potential changes that may have happened from the introduction of HFOT in our hospital compared to the years when this technique was not available. In analysing clinical practice, we aimed to assess the effectiveness of HFOT in patients with a diagnosis of bronchiolitis, as this is considered one of the leading causes of hospital admission in the first 2 years of life.11,12
The salient findings were an increase in the frequency of transfers and readmissions in the second period, despite there being no changes in the incidence of RSV or an increase in risk associated with age between the 2 periods. (Table 1). As described in the previous literature, the risk of transfer to the PICU was much higher in infants aged less than 3 months (11.5% compared to 1.9% in older children).2 In other studies, the proportion of admission to the PICU was similar to the proportion found in ours: González Martínez reported a proportion of 20%,13 Mayfield et al. a proportion of 13% of patients managed with HFOT14 and Franklin et al. a proportion of 11.7%.15 These changes could be due to a multi-annual oscillation phenomenon similar to the one described in patients with meningococcal sepsis, although Pelletier et al16 dismissed this hypothesis in a retrospective study in which they found no significant differences in the Pediatric Risk of Mortality (PRISM) or the Pediatric Logistic Organ Dysfunction (PELOD) scores.
It is surprising that as years passed, especially comparing the period before HFOT was available to the period after, there was an increase in the duration of oxygen therapy (0.88 días), from a mean of 1.52 días (SD, 2.04) in the first period to a mean of 2.40 days (SD, 2.42) in the second period, which suggests that the use of HFOT could be associated to an increased length of stay and the required days of oxygen therapy. This stood in contrast to the data published by other authors, such as Mc Kiernan et al.,8 Milani et al.17 or Riese et al.,18 although our data were closer to those published by Mace et al.19 or Riese et al.,20 who concluded that there was no difference in the length of stay in days when comparing HFOT with conventional oxygen therapy.
We found a substantial use of salbutamol, despite it not being currently a recommended treatment, although its use decreased over time, a trend that we also observed in the administration of adrenaline and hypertonic saline, which could be due to the progressive changes made to treatment protocols from the publication of the Spanish clinical practice guideline in 20109 to the NICE guideline published in 2015.21
As observed in other case series,1,2 RSV was detected in more than half of the patients. Other clinical characteristics of our patients were similar to those described in the previous literature.20,22
One of the limitations is that cardiorespiratory parameters were not recorded in the follow-up of all patients so that we could assess their clinical condition at the time respiratory support was prescribed or the transfer made. We also were not able to analyse the fraction of inspired oxygen (FiO2) maintained in patients during HFOT, so patients may have been hyperoxygenated or it may have been erroneously assumed that adequate peripheral oxygen saturations were maintained because a high FiO2. We were able to obtain follow-up data for the patients that were transferred, and found no differences in the severity threshold used to determine the need for referral.
Another of the limitations of the study, as has been noted in other works,23 is that it is based on the experience in a single facility and that the sample was small, in addition to the subjectivity in the prescription of HFOT, as the decision was made by the physician responsible for the patient at any given time, along with the lack of established severity thresholds to determine the indication or HFOT, the time to withdraw it or when HFOT has failed and the patient needs transfer to a PICU.
In spite of the above, the use of HFOT for respiratory support growing in frequency7 and becoming widespread in low-complexity units in general and our department in particular, probably due to the perceived comfort of the patients and the simplicity of the system, which is easy to use and requires little training and the same care procedures by nursing staff as the delivery of oxygen therapy with low-flow nasal prongs with monitoring with conventional pulse oximetry.
The HFOT system and interface seem to be well tolerated by patients and are easy to manage, so this could be considered an intermediate modality between low-flow oxygen therapy and non-invasive ventilation in a ward that currently does not have access to the latter modality.
Given that some authors, such as Kepreotes et al.24 and Franklin et al.,25 consider that the use of HFOT could achieve a reduction in the number of patients requiring admission to the PICU, while others, including Durand et al.26 and Modesto i Alapont et al.,27 did not find any benefits using this technique, additional evidence is required from either a randomised controlled trial, as proposed by Ramnarayan et al.,28 or a prospective study with a pre-established decision-making protocol for changes in respiratory support or patient transfer based on changes in cardiorespiratory parameters, the type and intensity of support (HFOT or conventional oxygen therapy, FiO2, flow rate, etc.) and severity scores to corroborate the findings of the study. We think that a protocol needs to be developed to standardise the use of HFOT in this group of patients, including repeated measurement of cardiorespiratory parameters or even performance of blood gas analysis or, if not possible, use of the oxygen saturation/ /FiO2 (S/F) during HFOT,29 and enhancing monitoring, with the use of techniques like transcutaneous carbon dioxide monitoring, to avoid prolonging the use of HFOT in patients that require a higher level of respiratory support.
While HFOT is easy to implement, it is a modality which, with little complication, requires close monitoring by nurses and physicians taking into account the predictors of failure described in the literature29–31 to ensure that HFOT is not prolonged without reassessing its appropriateness or delaying initiation of mechanical ventilation.
In conclusion, in our case review, most of the patients who required respiratory support were infants aged less than 5 months with RSV infection, and in recent years, there has been an increase in the use of HFOT and the need to transfer to the PICU. Although these results may not apply to other care settings, they do evince the need to pursue studies to assess the efficacy and effectiveness of HFOT for the management of disease of varying severity before its use is generalized.
FundingThe study did not receive any specific financial support from public, private or not-for-profit agencies.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Gutiérrez Moreno M, Barajas Sánchez V, Gil Rivas T, Hernández González N, Marugán Isabel VM, Ochoa-Sangrador C, Efectividad de la oxigenoterapia de alto flujo en hospital de segundo nivel en bronquiolitis, Anales de Pediatría. 2022;96:485–491.