The aim of our study is to evaluate whether the use of heliox (79:21) delivered through a low flow nasal cannula would improve respiratory distress in infants with acute bronchiolitis caused by RSV.
MethodsWe have conducted a prospective randomised controlled study. All patients fulfilling inclusion criteria were randomised to either heliox (79:21) or air via NC at 2L/min for a continuous 24h. Measurements were taken at baseline, after 2h and at the end of the 24h.
ResultsWe have included 104 patients into our study. The MCA-S did not show any significant difference between the two groups after 2h 4.3 vs. 4.1 (P=.78), or at 24h after 4.2 vs. 4.3 (P=.89). No difference was found in the proportion of participants progressed to MV, n-CPAP or oxygen via nasal cannula (RR 1.0, 0.86 and 0.89) (P=1.0, .77 and .73). There was no notable reduction in length of treatment in Heliox group 2.42 days vs. 2.79 days in air group P=.65. The oxygen saturation, PaO2, and PaCO2 did not have any statistical difference between the two studied groups after 2h and 24h of treatment.
ConclusionOur data showed absence of any beneficial effect of heliox in a concentration (79:21) delivered through low flow nasal cannula in terms of respiratory distress improvement in infants with RSV acute bronchiolitis.
El objetivo del estudio fue evaluar si el uso de heliox (79:21) administrado vía cánula nasal de bajo flujo mejora el trabajo respiratorio en lactantes con bronquiolitis aguda causada por VRS.
MétodosSe realizó un estudio prospectivo aleatorizado controlado. Todos los pacientes que cumplieron los criterios de inclusión se asignaron al azar a tratamiento con heliox (79:21) o con aire, administrados mediante cánula nasal a razón de 2L/min durante un período ininterrumpido de 24h. Se realizaron medidas basales, a las 2h de iniciar el tratamiento y al completarse las 24h.
ResultadosSe incluyeron 104 pacientes en el estudio. No se observaron diferencias significativas en la puntuación de la M-WCAS entre los dos grupos a las 2h (4,3 vs. 4,1; p=0,78) o al completarse las 24h (4,2 vs. 4,3; p=0,89). No hubo diferencias en las proporciones de participantes que progresaron a ventilación mecánica, CPAP-n u oxigenoterapia administrada mediante cánula nasal (RR: 1,0, 0,86 y 0,89; p=1,0, 0,77 y 0,73). No hubo una reducción significativa en la duración de tratamiento, de 2,42 días en el grupo tratado con heliox y de 2,79 días en el grupo tratado con aire (p=0,65). Tampoco hubo diferencias significativas entre los dos grupos bajo estudio en la saturación de oxígeno, PaO2 o PaCO2 a las 2 y a las 24h de tratamiento.
ConclusionesNuestros datos no mostraron ningún efecto beneficioso del heliox a una concentración de 79:21 administrado vía cánula nasal de bajo flujo en cuanto a la mejoría de la dificultad respiratoria en lactantes con bronquiolitis aguda por VRS.
Helium is an inert gas with a density lower than that of air. Therefore, carbon dioxide (CO2) can diffuse through helium more easily than through air.1 Helium enables easier maintenance of laminar flow with a reduction in turbulence in constricted airways, which in turn minimises resistance to inhaled gas.2 Therefore, breathing a mixture of oxygen and helium (heliox) will lower airway resistance to gas flow and subsequently decrease respiratory effort, especially in disorders associated with an increase in airway resistance.3 Accordingly, heliox can be used in patients suffering from upper airway obstruction,4 bronchiolitis or obstructive pulmonary diseases.5,6 In spontaneously breathing patients, heliox can be delivered through different interfaces, including facemasks with a reservoir bag inflated with a sufficient gas flow7 and connected to a semi-closed circuit that lowers heliox consumption to less than 1L/min, comparable to consumption with low-flow nasal cannula (LFNC).8 Heliox may be delivered invasively by adjusting the settings of mechanical ventilation.4
Respiratory syncytial virus (RSV) is an RNA virus that belongs to Paramyxoviridae family. After an incubation period that can last up to eight days, it initially replicates in the nasopharyngeal mucosa and then spreads to the epithelial lining of bronchioles, starting a lower respiratory tract infection.9 As the infection progresses, the epithelium becomes inflamed and produces an abundance of mucus, which is followed by cellular necrosis and then regeneration. Finally, small airways become obstructed, leading to air trapping and increased resistance of the lower airways.10
These properties have spurred a growing volume of research on the use of heliox as an adjuvant therapy aimed at improving oxygenation in mechanically ventilated patients with lower respiratory tract infection by RSV; however, the current evidence does not support the use of heliox in these patients.3
The aim of our study was to evaluate whether the use of heliox (79:21) delivered through (LFNC) would improve respiratory distress in infants with acute bronchiolitis caused by RSV.
MethodsParticipantsPatients eligible for participation in our study were infants aged one month to 2 years and admitted to the paediatrics ward with RSV acute bronchiolitis. The study was carried out between May 2015 and August 2016. The diagnosis of bronchiolitis was based on the presence of clinical criteria such as cough, tachypnoea, chest retractions, prolonged expiratory time, sibilant rhonci and hyperinflation of the lungs on chest radiography. The detection of respiratory syncytial virus as the aetiological agent of bronchiolitis was made by direct antigen detection assay in nasopharyngeal secretion samples. Patients were included into our study if they presented with manifestations of respiratory distress on admission but maintained oxygen saturations of 93% or higher without oxygen supplementation. After written informed consent was obtained from at least one of the legal guardians, patients that met the inclusion criteria were randomised to either the heliox group (79:21) or the air group (21%), with both treatments delivered via low-flow nasal cannulae at a flow of 2L/min for 24h without interruption.
We excluded infants who required supplemental oxygen or mechanical ventilation or with congenital heart defects (significant left-to-right shunt with or without pulmonary hypertension, or right-to-left shunt). Patients with known chronic lung disease, including bronchopulmonary dysplasia or diseases manifesting with airway hyperresponsiveness were also excluded. Failure to obtain informed consent also resulted in exclusion from the study.
Ethics and consent to participationThe study received the approval of the Institutional Research Board of the School of Medicine of Mansoura University, Egypt, under file no. R2/17.10.35.
Informed consent was obtained from at least one of the parents of each participant included in the study.
Study designWe conducted a prospective randomised controlled study. After obtaining written consent, all the enrolled participants were allocated to either Heliox (79:21) (Advanced Technology Company) or air delivered via LFNC at 2L/min for a continuous 24-h period by computer-based block randomisation. We divided participants into blocks of 4, ensuring that the variability within blocks was lesser than the variability between blocks. Then, subjects within each block were randomly assigned to treatment conditions.
In cases where at any point the oxygen saturation remained at 93% 90% for 10min or dropped below 90%, the infant was withdrawn from the trial and provided supplemental oxygen as prescribed by the treating physician.
The Modified Wood's Clinical Asthma Score (M-WCAS)11 is a tool used to assess variations in respiratory distress through time, with a maximum possible score of 11. Measurements of vital signs were taken at baseline and then hourly throughout the study, while the partial pressure of carbon dioxide (PaCO2), partial pressure of oxygen (PaO2) and the M-WCAS were measured and recorded at baseline, then at 1h intervals for 6h, and finally every 6h until treatment was discontinued.
RSV detectionThe presence of RSV was assessed by the TRU RSV test (Meridian Bioscience, Inc., USA) on nasopharyngeal samples. The TRU RSV is a qualitative capture immunoassay test for the rapid detection of RSV antigen in human samples. The test employs gold-linked monoclonal antibodies to RSV fusion protein and nucleoproteins (detector antibodies). Nasopharyngeal samples from study participants were collected with plastic shafted cotton swabs and submitted in a transport medium (0.85% saline) to the Microbiology Laboratory for processing. The test procedure was performed according to the directions provided by the manufacturer.
Measurements and outcomesBefore starting the study protocol, a chest X-ray was performed in each patient to assess for the presence of lung hyperinflation, defined as the diaphragm below the level of the 6th anterior thoracic rib. Arterial blood samples were drawn from all participants through arterial puncture following application of a topical anaesthetic cream to measure the PaCO2 and PaO2 at baseline and 2 and 24h after treatment initiation. The M-WCAS was recorded to assess variation in respiratory distress, at least 1h after any type of intervention, such as blood extraction, at baseline and 2 and 24h after treatment initiation. All patients were managed according to a standardised management protocol.10,12 Oxygen saturation was monitored continuously using a pulse oximeter (Masimo, Irvine, CA, USA). Other variables were also measured hourly for 6h and then every 6h for a total of 24h until heliox was discontinued. The obtained data were subsequently analysed.
Outcome measuresThe primary endpoint was the change in the level of respiratory distress as assessed by the M-WCAS at 2 and 24h after initiation of treatment. The secondary end points were the total duration of treatment required to improve respiratory distress for a period of 1h with the patient breathing room air and minimal respiratory effort (normal respiratory rate, without nasal flaring, tracheal tug, grunting, head bobbing, cyanosis, or use of accessory muscles except for mild intercostal retractions) and the proportion of patients in each treatment group that eventually required oxygen therapy or respiratory support.
Sample size calculationA sample size of 53 patients per arm would allow detection of a 0.75 point difference in the M-WCAS, assuming a standard deviation from the mean of 1.2 points, with a power of 90%, an α of 5% and a 0.05 level of significance using the independent samples t test (two-tailed).
Statistical analysisWe used the Mann–Whitney U test to compare the duration of treatment between the groups under study, and summarised the results as medians with their interquartile ranges (IQRs). We defined statistical significance as a P-value of less than 0.05. We used the chi square test to calculate relative risks. The population was described by means/proportions with their 95% confidence intervals. We performed survival analysis by the Kaplan–Meier method to analyse the duration of inpatient treatment in both groups. All the statistical analyses were performed using SPSS version 19.0 (Chicago, IL, USA).
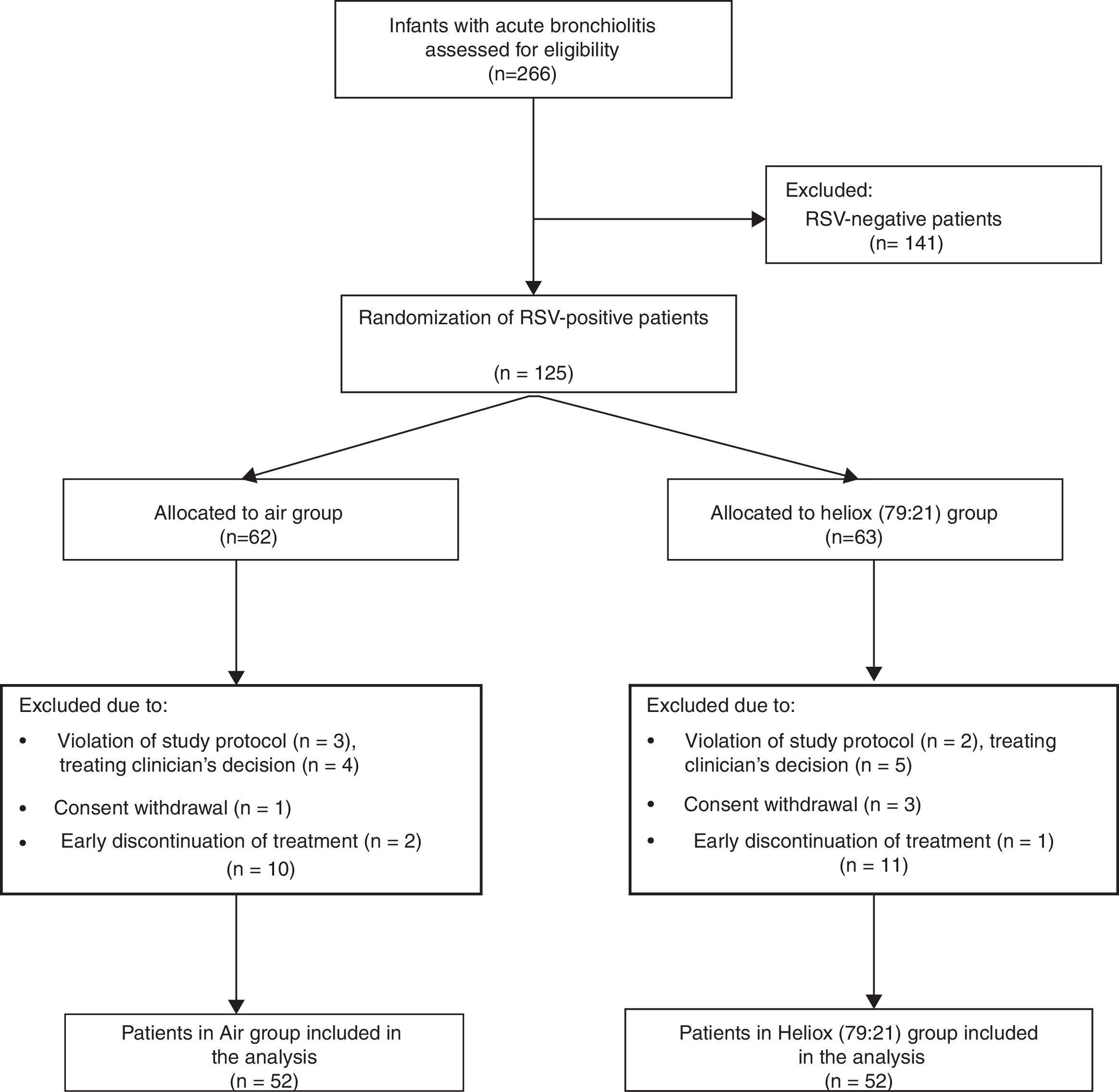
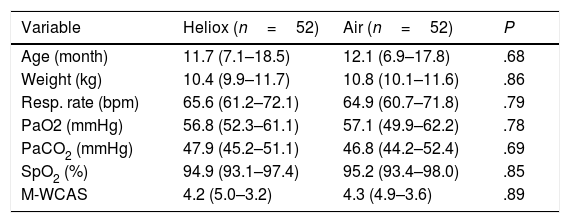
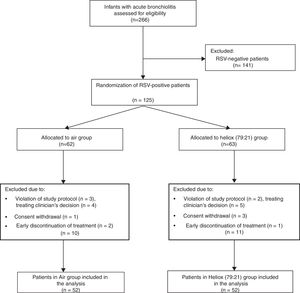
ResultsDuring the study period, 266 infants were admitted to our paediatrics department with acute bronchiolitis and assessed for eligibility. Only 104 patients were eventually allocated to a study protocol, with 52 patients included in each arm, as shown in the flow chart that summarises the flow of patients before and after randomisation (Fig. 1). All baseline measurements of study participants were similar in both groups, and are shown in Table 1.
Baseline characteristics of both groups.
| Variable | Heliox (n=52) | Air (n=52) | P |
|---|---|---|---|
| Age (month) | 11.7 (7.1–18.5) | 12.1 (6.9–17.8) | .68 |
| Weight (kg) | 10.4 (9.9–11.7) | 10.8 (10.1–11.6) | .86 |
| Resp. rate (bpm) | 65.6 (61.2–72.1) | 64.9 (60.7–71.8) | .79 |
| PaO2 (mmHg) | 56.8 (52.3–61.1) | 57.1 (49.9–62.2) | .78 |
| PaCO2 (mmHg) | 47.9 (45.2–51.1) | 46.8 (44.2–52.4) | .69 |
| SpO2 (%) | 94.9 (93.1–97.4) | 95.2 (93.4–98.0) | .85 |
| M-WCAS | 4.2 (5.0–3.2) | 4.3 (4.9–3.6) | .89 |
Modified Wood's Clinical Asthma Score (M-WCAS).
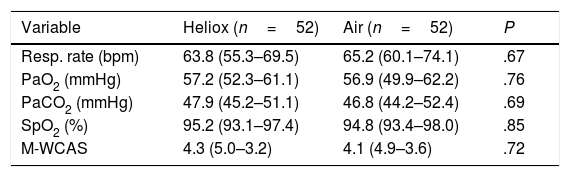
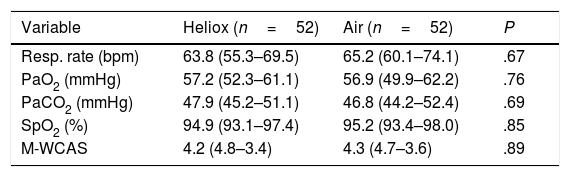
After 2h of treatment, there were no improvements in oxygen saturation or PaO2 in the heliox group compared to the air group (P=.85 and P=.76, respectively). Moreover, there was no significant difference in PaCO2 between the two groups (P=.69), as can be seen in Table 2. After 24h of treatment, there continued to be no statistically significant differences in oxygen saturation (P=.85), PaO2 (P=.76), and PaCO2 (P=.69) between the two groups under study, as shown in Table 3.
Differences between study groups 2h after initiation of treatment.
| Variable | Heliox (n=52) | Air (n=52) | P |
|---|---|---|---|
| Resp. rate (bpm) | 63.8 (55.3–69.5) | 65.2 (60.1–74.1) | .67 |
| PaO2 (mmHg) | 57.2 (52.3–61.1) | 56.9 (49.9–62.2) | .76 |
| PaCO2 (mmHg) | 47.9 (45.2–51.1) | 46.8 (44.2–52.4) | .69 |
| SpO2 (%) | 95.2 (93.1–97.4) | 94.8 (93.4–98.0) | .85 |
| M-WCAS | 4.3 (5.0–3.2) | 4.1 (4.9–3.6) | .72 |
Modified Wood's Clinical Asthma Score (M-WCAS).
Differences between study groups 24h after treatment initiation.
| Variable | Heliox (n=52) | Air (n=52) | P |
|---|---|---|---|
| Resp. rate (bpm) | 63.8 (55.3–69.5) | 65.2 (60.1–74.1) | .67 |
| PaO2 (mmHg) | 57.2 (52.3–61.1) | 56.9 (49.9–62.2) | .76 |
| PaCO2 (mmHg) | 47.9 (45.2–51.1) | 46.8 (44.2–52.4) | .69 |
| SpO2 (%) | 94.9 (93.1–97.4) | 95.2 (93.4–98.0) | .85 |
| M-WCAS | 4.2 (4.8–3.4) | 4.3 (4.7–3.6) | .89 |
Modified Wood's Clinical Asthma Score (M-WCAS).
The Modified Wood's Clinical Asthma Score also revealed no significant differences between the two groups after 2h of treatment (P=.78), and remained almost the same in both groups at the end of the 24h of treatment (P=.89), as can be seen in Table 3.
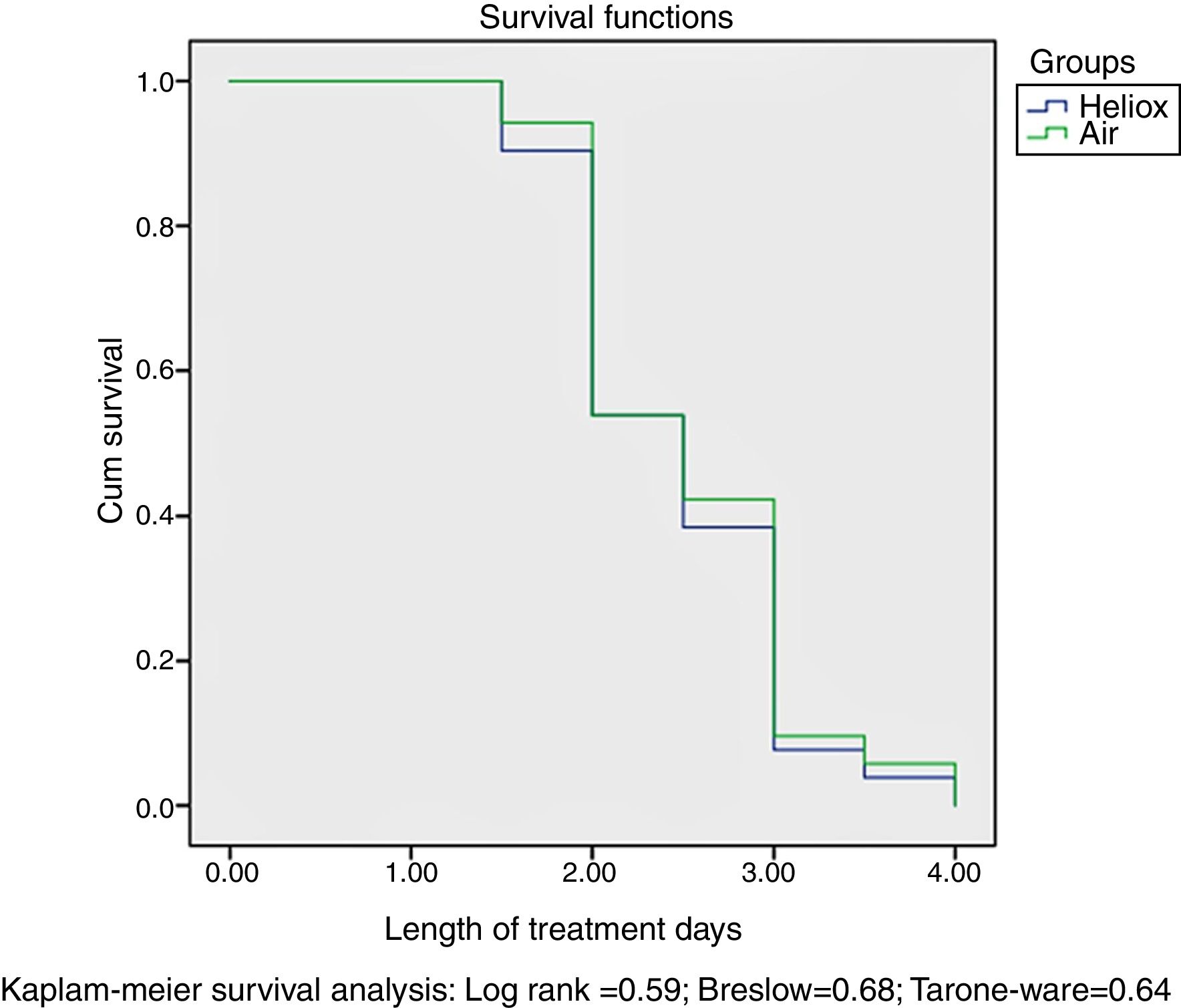
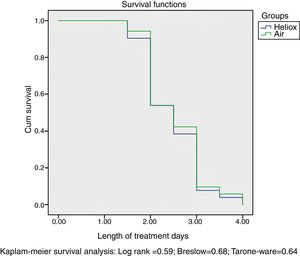
Our data found no differences between the two groups in the proportion of participants that eventually required mechanical ventilation, nasal continuous positive airway pressure (n-CPAP) or supplemental oxygen delivered by nasal cannula, as shown in Table 4. There was also no significant reduction in the duration of treatment in the heliox group compared to the air group (P=.65). Survival analysis by the Kaplan–Meier method did not find significant differences in the length of inpatient treatment between the heliox and the air groups, as seen in Fig. 2.
Proportion of participants that required respiratory support in the study groups.
| Variable n (%) | Heliox (n=52) | Air (n=52) | RR (95% CI) | P |
|---|---|---|---|---|
| Mechanical ventilation | 2 (3.8) | 2 (3.8) | 1.0 (0.14–6.8) | 1.0 |
| Nasal CPAP | 6 (11.5) | 7 (13.4) | 0.86 (0.31–2.4) | .77 |
| Oxygen via nasal cannula | 13 (25) | 14 (26.9) | 0.89 (0.47–1.7) | .73 |
There were no treatment-related adverse events. All patients recovered fully without sequelae.
DiscussionWhen helium is mixed with oxygen, it produces a mixture less dense than air13 that can reduce respiratory effort in airway obstruction by decreasing airway resistance and increasing alveolar ventilation.14–20
Our study showed that the use of helium mixed with air at a 79:21 concentration without addition of supplemental oxygen failed to achieve significant reductions in the clinical asthma score for assessment of respiratory distress or to improve oxygenation during the initial phase of treatment in patients with acute bronchiolitis caused by RSV. This finding is consistent with the work of Wurzel et al.,21 who reported the absence of any significant benefits within 24h of initiating heliox in outcomes including gas exchange, respiratory function, clinical respiratory scores or total length of stay.
Nevertheless, our previous study using helium with 30% supplemental oxygen (70:30) delivered by high-flow nasal cannula (HFNC) found a brief improvement in oxygenation during the initial phase of treatment in patients with acute bronchiolitis by RSV that declined rapidly after 2h of treatment. This improvement in oxygenation with a heliox mixture of (70:30) during initial period of the intervention that we previously reported could be explained by the delivery of a higher concentration of oxygen and the mechanism of action, which facilitates the flow of oxygen through narrow airways. It may also be due to the effect of the respiratory support provided through HFNC.22 Similarly, a recent Cochrane review by Moraa et al. presented data on the short-lived benefits of the administration of heliox in children with moderate to severe croup when combined with oral or intramuscular dexamethasone.4 Other authors attributed such short-lived improvements to the concomitant use of non-invasive ventilation (n-CPAP) rather than the effect of heliox itself.19 The absence of an increase in CO2 elimination in patients with bronchiolitis caused by RSV in our study was consistent with the findings of our previous study on the use of heliox (30:70) combined with HFNC,22 and has also been reported by Gross et al.,3 who found no beneficial effect of different heliox mixtures on PaCO2 in mechanically ventilated infants with moderate to severe lower respiratory tract disease caused by RSV. Kneyber et al. have reported similar findings, although the participants in their study had more severe disease compared to ours, with a higher PaCO2.23 Therefore, it is important to keep in mind that heliox has no direct treatment effects and is only a temporising measure until definitive therapies take effect or the disease process resolves.24 Thus, should be only considered as an adjunct therapy in the management of severe airway obstruction.
In our study, heliox failed to achieve any notable reduction in the proportion of participants who progressed to mechanical ventilation, n-CPAP or supplemental oxygen. Chowdhury et al. have reported similar results in patients with non-RSV bronchiolitis with the use of heliox at the same concentration delivered through LFNC.25 Furthermore, we did not find a reduction in the length of hospitalisation in RSV-positive children with bronchiolitis treated with heliox, contrary to the findings of the aforementioned study by Chowdhury and colleagues, who reported a reduction of nearly 50% in the number of treatment days compared to children treated with air.25
There were no treatment-related adverse events. All patients recovered fully and without sequelae.
None of our participants, irrespective of the treatment received, suffered from any adverse effects, and heliox was well tolerated. However, we ought to mention some limitations in our study. Heliox was administered through low-flow nasal cannulae, which are not the best interface for delivering the gas mixture, as high-flow nasal cannulae, n-CPAP and mechanical ventilation are better methods for its administration. Also, differences between gas delivery systems made randomisation not blind, while the small sample size did not allow us to discriminate between clinical subtypes based on severity, aspects that should be the subject of further research.
ConclusionsOur data showed no beneficial effect of adding helium to air in terms of reducing respiratory effort in infants with acute bronchiolitis caused by RSV. Therefore, the growing evidence on the efficacy of heliox in reducing respiratory effort has to be interpreted carefully, as it remains unclear whether heliox therapy is or not beneficial in the context of treatment of acute bronchiolitis due to RSV.
Conflict of interestThe authors declare that they have no conflict of interest.
Please cite this article as: Seliem W, Sultan AM. ¿Mejora la administración de helio mediante cánula nasal de bajo flujo la dificultad respiratoria en lactantes con bronquiolitis aguda por virus respiratorio sincitial? Estudio aleatorizado controlado. An Pediatr (Barc). 2019;90:3–9.