To determine the correlation between the findings seen in early lung ultrasound with the clinical severity scales, and its association with the subsequent progression of the mild–moderate acute bronchiolitis (AB).
Patients and methodsAn observational prospective study conducted on infants with mild–moderate AB, using lung ultrasound in the first 24h of hospital care. The lung involvement was graded (range 0–50 points) based on an ultrasound score (ScECO) and 2 routinely used clinical scales: the modified Wood Downes Ferres (WDFM), and the Hospital Sant Joan de Deu (HSJD). The relationship between the ScECO and the subsequent clinical progression (admission to the Paediatric Intensive Care Unit (PICU), days in hospital, and days of oxygen therapy) was also determined.
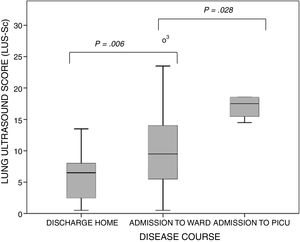
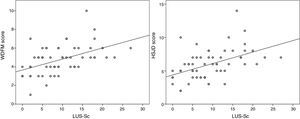
ResultsThe study included a total of 59 patients, with a median age of 90 days (IQR: 30–270 days). The median ScECO score was 6 points (2–8) in the patients that did not require hospital admission, with 9 points (5–13.7) admitted to the ward, and 17 (14.5–18) in the patients who needed to be transferred from the ward to the PICU (P=.001). The ScECO had a moderate lineal association with the WDFM scale (rho=0.504, P<.001) and the HSJD (rho=0.518; P<.001). The ScECO was associated with admission to PICU [OR 2.5 (95% CI: 1.1–5.9); P=.035], longer hospital stay [1.2 days (95% CI: 0.55–1.86); P=.001] and duration of oxygen therapy [0.87 days (95% CI: 0.26–1.48); P=.006].
ConclusionsThere is a moderate correlation between early lung ultrasound findings with the severity of the AB evaluated by the clinical scales, as well as some relationship with the clinical progression.
Evaluar la correlación entre hallazgos de la ecografía pulmonar realizada precozmente con las escalas de gravedad clínica y su asociación con la evolución posterior en la bronquiolitis aguda (BA) leve-moderada.
Pacientes y métodosEstudio observacional prospectivo. Se incluyó a lactantes con BA leve-moderada evaluados mediante ecografía pulmonar en las primeras 24 h tras la atención hospitalaria. Se graduó la afectación pulmonar (rango 0-50 puntos) sobre la base de un score ecográfico (ScECO). Se evaluó la correlación entre el ScECO y 2 escalas clínicas de uso habitual: escala de Wood Downes Ferres modificada (WDFM) y escala del Hospital Sant Joan de Déu (HSJD). Así mismo se valoró la asociación entre el ScECO y la evolución clínica posterior (ingreso en la Unidad de Cuidados Intensivos Pediátricos [UCIP], días de hospitalización y días de oxigenoterapia).
ResultadosSe incluyó a 59 pacientes con una edad mediana de 90 días (RIQ: 30-270 días). La puntuación mediana del ScECO fue de 6 puntos (2-8) en los pacientes que no requirieron ingreso, 9 (5-13,7) en los ingresados en planta y 17 (14,5-18) en los pacientes que precisaron traslado de planta a la UCIP (p=0,001). El ScECO tuvo una correlación lineal moderada con la escala de WDFM (rho=0,504, p<0,001) y HSJD (rho=0,518; p <0,001). El ScECO se asoció al ingreso en UCIP (OR 2,5 [IC del 95%: 1,1-5,9]; p=0,035), mayor estancia hospitalaria (1,2 días [IC del 95%: 0,55, 1,86]; p=0,001) y duración de oxigenoterapia (0,87 días [IC del 95%: 0,26, 1,48]; p=0,006).
ConclusionesLa ecografía pulmonar precoz se correlaciona de forma moderada con la gravedad de la BA evaluada por escalas clínicas y guarda cierta relación con la evolución clínica.
Acute bronchiolitis (AB) is the most frequent reason for hospital admission in infants, constituting a significant health care and economic burden. Although in most cases AB has a benign course, a subset of cases progress to severe disease requiring admission to the paediatric intensive care unit (PICU) and respiratory support, which are associated with a considerable morbidity and mortality. It is often difficult to predict which patients will have this adverse progression, which results in the need for prolonged inpatient monitoring in certain at-risk groups. Clinical severity scales are widely used and can be helpful to make an objective and reproducible assessment of the course of the disease and the response to treatment. However, there is insufficient evidence of their usefulness in predicting the outcomes of mild-to-moderate AB.1–3 The availability of a point-of-care tool allowing us to predict which patients may have an unfavourable course would be very useful for the purpose of preventing adverse events, intensifying monitoring and optimising the use of resources (transfer to hospitals with a PICU, early initiation of respiratory support, etc.).
Lung ultrasound allows the assessment of the extent of pleuropulmonary involvement at the bedside easily and readily and without adverse effects. It has been proven to be useful and in many instances superior to chest radiography in the diagnosis of transient tachypnoea of the newborn, acute respiratory distress syndrome (ARDS), pneumonia, pleural effusion and pneumothorax.4–11 Despite the extensive evidence gathered on other acute pulmonary diseases, few studies have assessed the usefulness of lung ultrasound in AB. Studies published to date agree that the extent of lung lesions is associated with the severity of AB.12–14 It would thus be sound to hypothesise that the extent of lung involvement could be a valuable predictor of the clinical course of AB, as is the case in other diseases such as ARDS, in which it is of proven utility in predicting mortality or response to treatments such as prone ventilation or recruitment maneouvres.15–20 However, no study to date has assessed the usefulness of early lung ultrasound in predicting the clinical course of AB.
We had 2 main objectives in conducting this study: on the one hand, to analyse the association between lung ultrasound findings and severity in mild-to-moderate AB as assessed by 2 clinical severity scores used in our region, and on the other to analyse whether early lung ultrasound findings are associated with clinical outcomes in patients presenting to hospital with mild-to-moderate AB.
Patients and methodsSampleWe conducted a prospective observational study between October 2016 and March 2017. We defined AB as an episode of respiratory distress characterised by manifestations of lower respiratory tract involvement (wheezing, rales) following an upper respiratory tract infection in infants aged less than 12 months, whether or not this was the first episode of the kind.1,2 We included patients aged less than 1 year with a clinical diagnosis of mild-to-moderate AB managed in our hospital as long as the lung ultrasound examination was performed by the researchers in the first 24h of care. We excluded patients that presented to the hospital with severe AB (Wood-Downes score modified by Ferrés [MWDF]>8 and Hospital Sant Joan de Déu score [HSJD]>10), patients admitted to the PICU in the first 24h of care, patients with a diagnosis of pneumonia based on sonographic findings, patients with haemodynamically significant congenital heart disease and patients born preterm with a history of bronchopulmonary dysplasia, whose ultrasound features are very similar to those of AB.
MeasuresWe collected demographic and clinical data for each patient at the time of arrival to hospital. The clinical assessment of the patients included measurement of vital signs and oxygen saturation and calculation of 2 clinical severity scores commonly used in our region (MWDF and HSJD scores) by the physician in charge.21,22 We analysed the clinical outcomes of patients in the month that followed their visit to our hospital (return to emergency department, hospital admission or development of early complications) through the review of electronic health records and telephone calls. We followed up patients admitted to hospital until they were discharged, recording the treatments they received and their clinical outcomes. The primary endpoints were the need for PICU admission, use of invasive mechanical ventilation, length of stay and duration of oxygen therapy. The main criterion for PICU admission was the development of severe respiratory distress, diagnosed based on clinical severity scores. Other criteria used to determine transfer to the PICU were apnoeic episodes, significant hypoxaemia (need of supplemental oxygen at concentrations of more than 50% to achieve a saturation greater than 90%) or hypercapnia.
Lung ultrasoundIn adherence with the protocol of the study, all participants underwent a lung ultrasound examination within 24h of arrival to the hospital.
The examination was performed with a portable ultrasound machine (SonoSite M-Turbo, Fujifilm, Japan) and a 6–13MHz linear array probe in all patients. Two paediatrics resident physicians (observer A and observer B) with previous experience in lung ultrasound performed the lung ultrasound examinations and interpreted their findings. These resident physicians had performed more than 25 lung ultrasound examinations in their clinical practice (mainly for diagnosis of pneumonia and its complications). They received specific training for this study delivered in two 2-h-long theoretical/practical workshops that included a review and discussion of the sonographic features of AB and performance under supervision of at least 10 lung ultrasound examinations in patients with AB (which were not included in the study).23–25
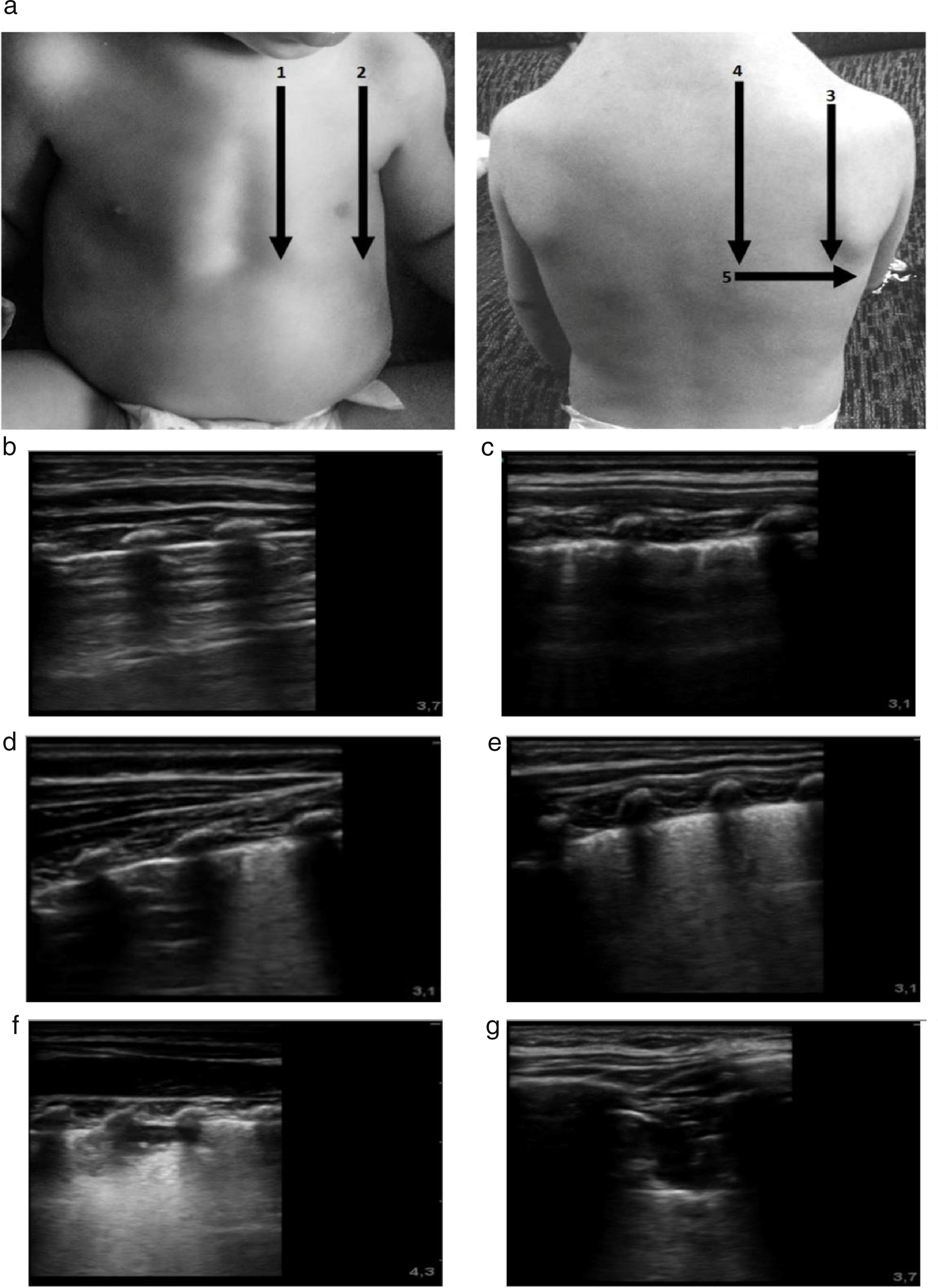
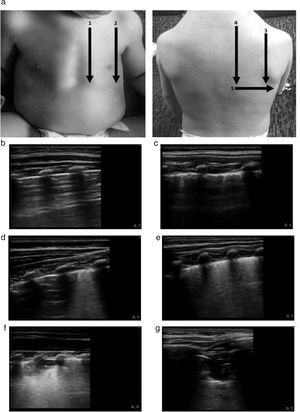
The imaging protocol included the examination of 10 chest zones (5 per hemithorax), capturing a 10-s video clip of each zone (Fig. 1). All images were recorded digitally for later analysis. The images were analysed in random order, and the evaluators were blinded to the clinical data of the patient to ensure an objective and unbiased interpretation. Three different elements of lung involvement in AB were assessed in each zone: pleural line abnormalities (PAs), extent of alveolar interstitial syndrome (AIS) and presence of subpleural lung consolidations (SCs). Pleural line abnormalities were defined as pleural thickening or loss of lung sliding; and graded with a score of 0 points (absence of abnormalities) or 1 point (presence of abnormality) adding to a possible maximum of 10 points for all zones. Interstitial syndrome was graded with a score of 0 points (normal pattern with a predominance of A lines), 1 point (AIS with converging B lines, defined as 3 or more B lines or white lung, in 1 intercostal space) or 2 points (AIS with convergent B lines, defined as 3 or more B lines per intercostal space or white lung, in 2 adjacent intercostal spaces) with a possible maximum of 20 points. Subpleural consolidation was graded with a score of 0 points (absent), 1 point (consolidation<1cm), and 2 points (consolidation<1cm or multiple consolidations) with a possible maximum of 20 points. We calculated the total lung ultrasound score (LUS-Sc) adding the scores for each individual zone. Thus, the maximum possible total score was 50 points (range, 0–50) (Fig. 1).
(a) Protocol for lung ultrasound acquisition and interpretation. The scanned zones are marked with numbers. The ultrasound score is noted in the images labelled with letters. The images show the score assigned to each detected abnormality. (b) Normal lung: 0 points. (c) Pleural thickening. LUS-Sc: 1 point. (d) Focal AIS focal. LUS-Sc: 1 point. (e) Diffuse AIS. LUS-Sc: 2 points. (f) SCs<1cm. LUS-Sc: 1 point. (g) SCs>1cm. LUS-Sc: 2 points. AIS, alveolar interstitial syndrome; SC, subpleural consolidation; 1: anterior parasternal zone; 2: anterior axillary zone; 3: posterior axillary zone; 4 posterior paravertebral zone; 5: posterior linea scapularis zone.
We summarised the data using the median and interquartile range, and absolute frequencies and percentages. In a first analysis, we assessed the interrater reliability between the paediatricians trained to perform the lung ultrasound examinations and a paediatrician with more than 5 years’ experience in the technique. We analysed the agreement in the assessment of PAs, AIS and the presence and extent of SCs in thirty 10-s clips randomly chosen from all the ultrasound examinations of patients with AB. We used the Cohen kappa coefficient to measure agreement, and considered agreement substantial if kappa was greater than 0.8 and moderate if kappa was greater than 0.6, applying the thresholds proposed by Landi and Koch. We used the intraclass correlation coefficient to assess the degree of interrater agreement. We then analysed the association between the LUS-Sc (total score and PA, AIS and SC subscores) and the clinical parameters of AB severity using the Spearman correlation coefficient. Last of all, we used multivariate regression to assess for the presence of an independent association between the LUS-Sc and the need for admission to the PICU, the length of stay and the duration of oxygen therapy. We considered P-values of less than 0.05 statistically significant. We performed the statistical analysis with the software SPSS version 22.0.
Ethical considerationsThe study protocol was evaluated and approved by the Clinical Research Ethics Committee, and the parents or legal guardians of participants provided their written informed consent to their participation in the study and the publication of its results.
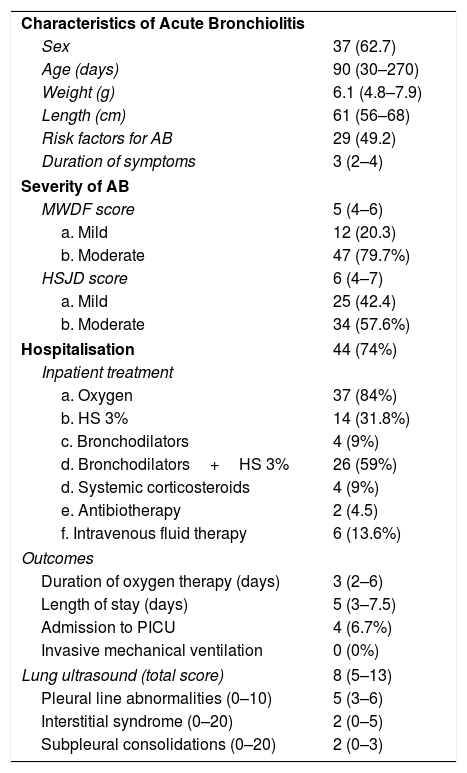
ResultsWe included 59 patients with mild-to-moderate AB with a median age of 90 days (IQR, 30–270) and a median weight of 6.1kg (IQR, 4.8–7.9). Forty-four patients required hospital admission for a median length of stay of 5 days (IQR, 3–7.5), and 37 (84%) required oxygen therapy for a median of 3 days (IQR, 2–6). The lung ultrasound examination was performed at the time of admission to the paediatric ward in 41 patients, and in the emergency department in 18. Four patients (6.7%) were admitted to the PICU, and none required invasive mechanical ventilation. Table 1 summarises the characteristics of the patients, and Appendix B presents supplementary material.
Patient characteristics.
| Characteristics of Acute Bronchiolitis | |
| Sex | 37 (62.7) |
| Age (days) | 90 (30–270) |
| Weight (g) | 6.1 (4.8–7.9) |
| Length (cm) | 61 (56–68) |
| Risk factors for AB | 29 (49.2) |
| Duration of symptoms | 3 (2–4) |
| Severity of AB | |
| MWDF score | 5 (4–6) |
| a. Mild | 12 (20.3) |
| b. Moderate | 47 (79.7%) |
| HSJD score | 6 (4–7) |
| a. Mild | 25 (42.4) |
| b. Moderate | 34 (57.6%) |
| Hospitalisation | 44 (74%) |
| Inpatient treatment | |
| a. Oxygen | 37 (84%) |
| b. HS 3% | 14 (31.8%) |
| c. Bronchodilators | 4 (9%) |
| d. Bronchodilators+HS 3% | 26 (59%) |
| d. Systemic corticosteroids | 4 (9%) |
| e. Antibiotherapy | 2 (4.5) |
| f. Intravenous fluid therapy | 6 (13.6%) |
| Outcomes | |
| Duration of oxygen therapy (days) | 3 (2–6) |
| Length of stay (days) | 5 (3–7.5) |
| Admission to PICU | 4 (6.7%) |
| Invasive mechanical ventilation | 0 (0%) |
| Lung ultrasound (total score) | 8 (5–13) |
| Pleural line abnormalities (0–10) | 5 (3–6) |
| Interstitial syndrome (0–20) | 2 (0–5) |
| Subpleural consolidations (0–20) | 2 (0–3) |
Risk factors for AB: age<6 weeks, preterm birth<35 weeks’ gestation, chronic cardiac or respiratory disease, immunodeficiency and absence of exclusive breastfeeding.
HS, hypertonic saline.
The interrater agreement between the paediatricians that performed and interpreted the ultrasound examinations and a paediatrician experienced in lung ultrasound was substantial for the assessment of PAs (observer A kappa, 0.851; observer B kappa, 0.841) and of AIS (observer A kappa, 0.836; observer B kappa, 0.877) and moderate for the assessment of SCs (observer A kappa, 0.620; observer B kappa, 0.695). The interrater intraclass correlation coefficient (absolute agreement between individual measurements) for the total score in the LUS-Sc was 0.917 (95% CI, 0.854–0.956).
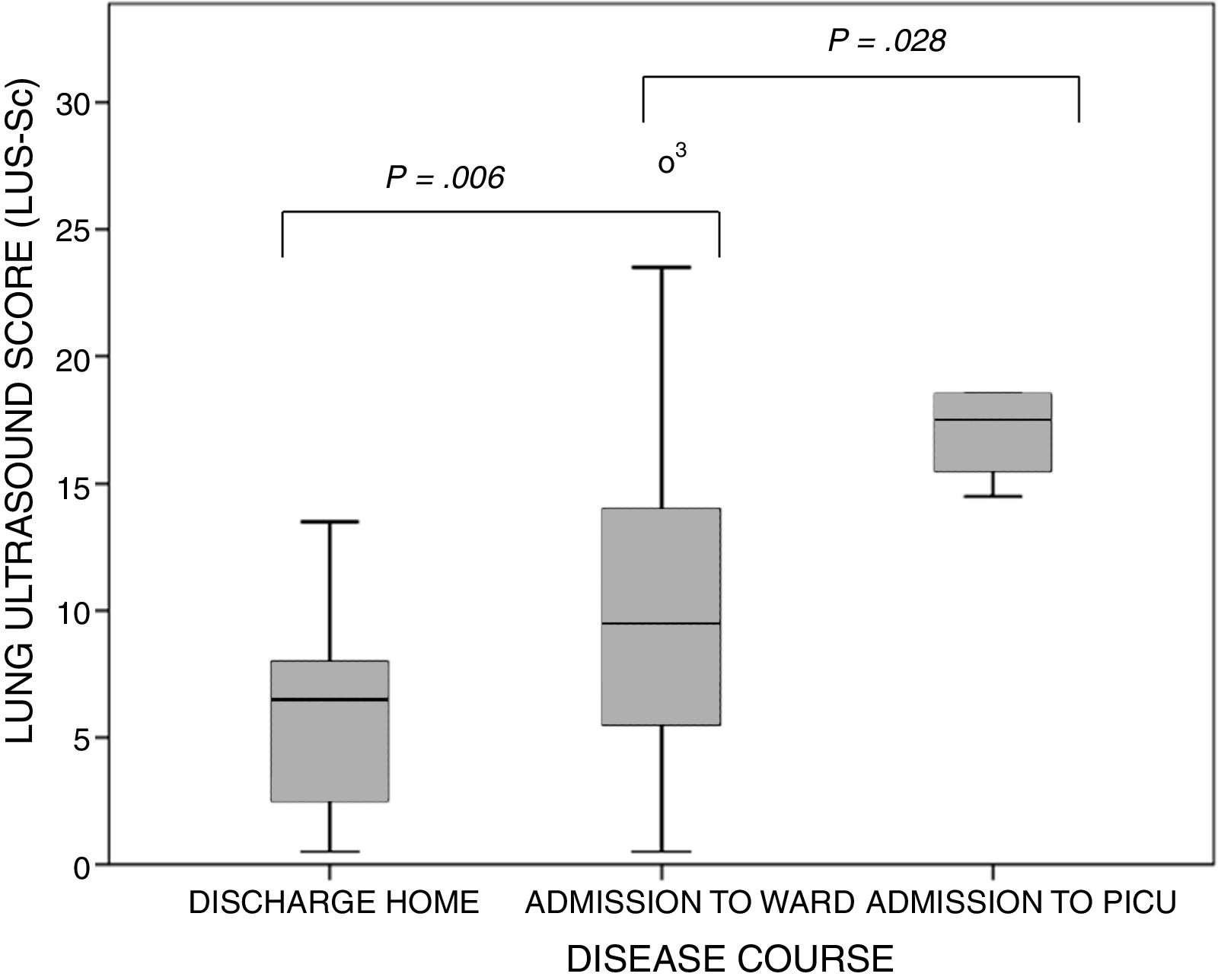
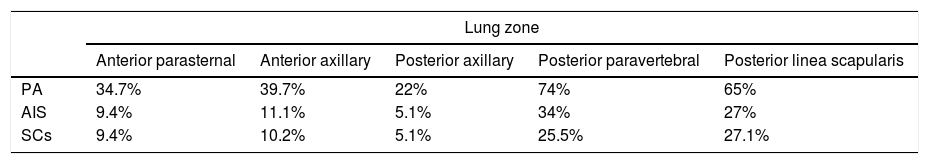
The ultrasound examinations were performed a median of 3 days (IQR, 2–4) from the onset of symptoms of AB. None of the patients were receiving respiratory support before the examination (except for conventional support with supplemental oxygen delivered via nasal prongs). The lung zones affected most frequently were the posterior paravertebral and the linea scapularis zones (Table 2). The median score in the LUS-Sc was 8 points (IQR, 5–13) in the overall sample, 6 points (IQR, 2–8) in the subset of patients discharged home from the emergency department, 9 points (IQR, 5–13.7) in the subset admitted to the paediatric ward and 17 points (IQR, 14.5–18) in the subset admitted to the PICU (P<.001) (Fig. 2). We also found statistically significant differences in the degree of AIS (discharge home, 0 [IQR, 0–2]; admission to ward, 4.5 [IQR, 3–6]; transfer to PICU, 5.5 [IQR, 5–6]; P<.001) and of SCs (discharge home, 0 [IQR, 0–2]; admission to ward, 2 [IQR, 0–3.7]; transfer to PICU, 5 [IQR, 4.2–5.7]; P=.004), but not in the severity of PAs. We did not find significant differences in severity between infants aged less than 3 months and older infants: MWDF score of 5 (IQR, 4–6) vs 4.5 (IQR, 4–7) (P=.459), and HSJD score of 6 (IQR, 4–7) vs 5.5 (IQR, 4–7) (P=.662). However, the LUS-Sc scores were higher in infants aged less than 3 months: 9 (IQR, 6–13) vs 7.5 (IQR, 2–13) (P=.069), with a higher AIS subscore (3 [IQR, 1–5] vs 1 [IQR, 0–4.5]; P=.127), and more SCs (4.5 [IQR, 3–6] vs 2 [IQR, 0–4]; P=.106), although these differences were not statistically significant.
Relative frequency of lung involvement detected by ultrasound in the assessed lung zones (the mean of both lungs is showed for each zone).
| Lung zone | |||||
|---|---|---|---|---|---|
| Anterior parasternal | Anterior axillary | Posterior axillary | Posterior paravertebral | Posterior linea scapularis | |
| PA | 34.7% | 39.7% | 22% | 74% | 65% |
| AIS | 9.4% | 11.1% | 5.1% | 34% | 27% |
| SCs | 9.4% | 10.2% | 5.1% | 25.5% | 27.1% |
AIS, alveolar interstitial syndrome; PA, pleural line abnormality; SC, subpleural consolidation.
Boxplots of the distribution of the values of the lung ultrasound score by course of disease category. The bars indicate the comparison of subset pairs (discharge home vs admission to ward and admission to ward vs admission to PICU) in the multiple comparison analysis. P=.001 (Kruskal–Wallis test).
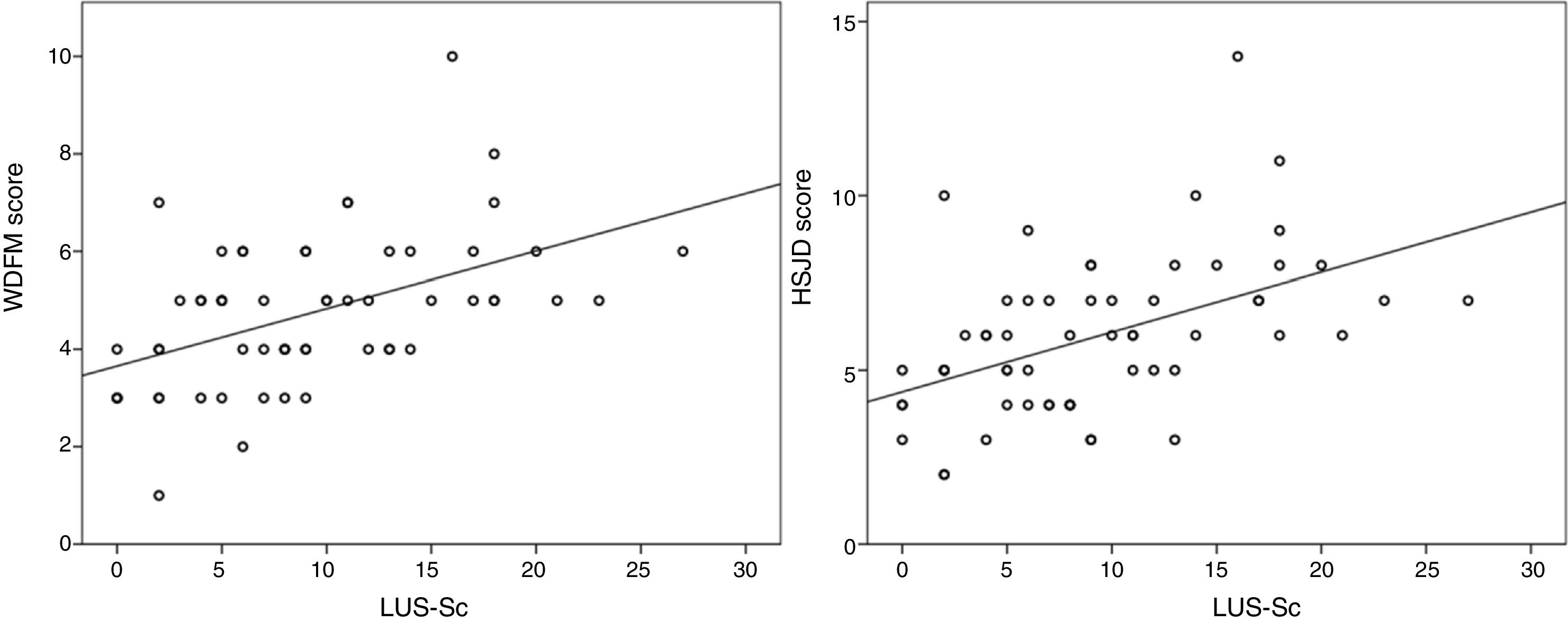
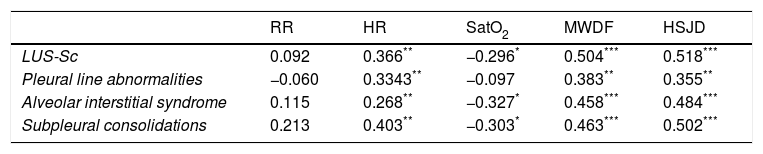
We found a statistically significant correlation between the LUS-Sc scores, especially the degree of AIS and SCs, and all clinical parameters except the respiratory rate (Table 3). The strongest correlations were between the total LUS-Sc score and the scores in the MWDF (Spearman rho, 0.504, P<.001) and the HSJD scales (Spearman rho, 0.518; P<.001). Fig. 3 presents the scatterplots for these correlations.
Spearman correlation coefficients for the association of lung ultrasound findings and clinical parameters. Value of the clinical parameters relative to the median lung ultrasound score.
| RR | HR | SatO2 | MWDF | HSJD | |
|---|---|---|---|---|---|
| LUS-Sc | 0.092 | 0.366** | −0.296* | 0.504*** | 0.518*** |
| Pleural line abnormalities | −0.060 | 0.3343** | −0.097 | 0.383** | 0.355** |
| Alveolar interstitial syndrome | 0.115 | 0.268** | −0.327* | 0.458*** | 0.484*** |
| Subpleural consolidations | 0.213 | 0.403** | −0.303* | 0.463*** | 0.502*** |
| RR | HR | SatO2 | MWDF | HSJD | |
|---|---|---|---|---|---|
| LUS-Sc | |||||
| <8 points | 53 (42–62) | 143 (130–160) | 96 (94–98) | 4 (3–5) | 5 (4–6) |
| ≥8 points | 54 (44–60) | 160 (144–172)* | 93 (90–98) | 5 (4–6)* | 7 (5–8)* |
HR, heart rate; HSJD, Hospital Sant Joan de Déu score; MWDF, Wood Downes score modified by Ferrés; RR, respiratory rate; SatO2, oxygen saturation.
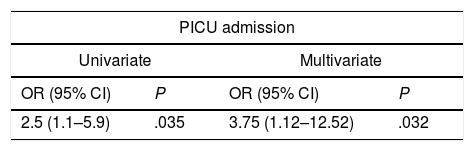
For every 5-point increase in the LUS-Sc, there were increases in the probability of admission to the PICU (OR, 2.5 [95% CI, 1.1–5.9]; P=.035), in length of stay by 1.2 days (95% CI, 0.55–1.86; P<.001) and in duration of oxygen therapy by 0.87 days (95%CI, 0.26–1.48; P=.006). These associations remained significant after adjusting for sex, weight, age, duration of AB symptoms and the presence of risk factors for severe AB (Table 4).
Association between the total lung ultrasound score and clinical endpoints in the multivariate analysis.
| PICU admission | |||
|---|---|---|---|
| Univariate | Multivariate | ||
| OR (95% CI) | P | OR (95% CI) | P |
| 2.5 (1.1–5.9) | .035 | 3.75 (1.12–12.52) | .032 |
| Length of stay (days) | |||
|---|---|---|---|
| Univariate | Multivariate | ||
| B coefficient (95% CI) | P | B coefficient (95% CI) | P |
| 1.2 (0.55. 1.86) | <.001 | 1.09 (0.29–1.79) | .003 |
| Days of oxygen therapy | |||
|---|---|---|---|
| Univariate | Multivariate | ||
| B coefficient (95% CI) | P | B coefficient (95% CI) | P |
| 0.87 (0.26–1.48) | .006 | 0.75 (0.10–1.4) | .024 |
We assessed the association between the ultrasound score and admission to the PICU by logistic regression, and the association of the ultrasound score and the length of stay and the duration of oxygen therapy with linear regression. We present the ORs and B coefficients for 5-point increases in the ultrasound score with the corresponding 95% confidence intervals and P-values. The model was adjusted for sex, weight, age, duration of AB and presence of risk factors for severe AB (age<6 weeks, preterm birth<35 weeks’ gestation, underlying chronic disease or absence of exclusive breastfeeding).
In this preliminary study, we found that lung ultrasound findings were correlated to symptom severity in mild-to-moderate AB and had a certain value in the prediction of the subsequent course of the disease. However, our findings could not prove that they added to the information provided by clinical severity scores for the purpose of anticipating an unfavourable progression.
Very few studies have used lung ultrasound in the context of AB, despite it being a method widely used in the assessment of patients with acute pulmonary diseases such as pneumonia, pleural effusion or lung oedema, in which its diagnostic yield exceeds that of plain X-rays while avoiding exposure to ionising radiation.5–7,12
In 2011, in a study comparing ultrasound and X-ray findings in patients with AB, Caiulo et al. found that the extent of SCs and AIS was associated with disease severity, and that the number and grading of SCs paralleled the scores in the Downes scale.12 In 2015, Basile et al. assessed the usefulness of ultrasound abnormalities in patients with AB, in terms of the presence and extent of SCs and the degree of AIS, for identification of patients requiring oxygen therapy.13 Our results were consistent with those of these studies, and we have also found evidence that abnormal ultrasound features exhibit a similar linear correlation with 2 validated clinical severity scores.26
We ought to mention that our study did not find a linear correlation between respiratory rate (which is widely considered a sensitive marker of respiratory involvement) and lung ultrasound findings. We do not have an obvious explanation for this result. It is possible that the greater variability and the lack of specificity of respiratory rate (due to its association with fever, nasal obstruction, agitation in the patient, etc.) combined with the small sample size contributed to this unexpected result.
Ultrasound findings such as PAs, SCs or the presence of AIS are not specific for AB and overlap significantly with the findings in other diseases with diffuse interstitial involvement, such as bronchopulmonary dysplasia or ARDS.6,27 However, our study and others in the previous literature are a proof of concept and suggest that the degree of sonographic abnormality reflects the severity of the pathophysiological process underlying AB to some extent. We know that the most severe cases of AB are those with presentations similar to that of ARDS, with severe interstitial involvement and a decrease in lung compliance as opposed to a pattern of obstruction and air trapping. It is possible that the early detection of reduced aeration by lung ultrasound could be useful in predicting the course of disease. In our study, we observed that patients that ended up requiring admission to PICU had more extensive involvement in the early ultrasound examination compared to patients discharged home or admitted exclusively to the paediatric ward, and also found that ultrasound was of some value in identifying patients that eventually required longer lengths of stay and more days of oxygen therapy. However, our statistical models did not show that these associations remained significant after adjusting for the clinical severity scores. This is not sufficient reason to definitely rule out the use of ultrasound in AB, but should rather encourage further research on its potential role in this disease. For instance, we observed that in patients with a similar severity determined on the basis of clinical features, ultrasound revealed a greater compromise of lung aeration in infants aged less than 3 months compared to older infants, which could contribute to the more severe course of disease observed in the younger set, in whom alveolar and interstitial involvement may be more prevalent, contrary to the predominance of obstruction and air trapping in older infants.
All current clinical practice guidelines agree that routine use of diagnostic tests is not recommended in AB.1,2 However, ultrasound examination could be useful in cases where despite performing the routine clinical assessment the need for inpatient observation remains unclear. Sonographic evidence of extensive involvement should alert us to the possibility of progression to severe disease. Thus, in our study, a LUS-Sc score of more than 13.5 points had a sensitivity of 100% and a specificity of 82% in predicting admission to the PICU.
LimitationsThe findings of our study must be interpreted taking into account its limitations. The ultrasound examination was performed a median of 3 days after the onset of AB, and while this is the time when symptoms usually peak, it is possible that there were differences between patients in the degree of lung involvement and that the administration of aerosol therapy affected the sonographic findings. However, there is no evidence in the literature that bronchodilators modify the course of disease or the sonographic features of lung involvement in AB. Our study included few patients, who also had relatively low scores (median of 8 points) in an ultrasound-based scale with a maximum possible score of 50 points. This may have limited the power of our study to detect an independent association between ultrasound findings and course of disease, which in the epidemic season under study tended to be relatively benign (short lengths of stay and few admissions to the PICU).
The ultrasound score used in our study assessed more zones per lung than previous studies, which may affect its reproducibility and applicability to clinical practice. Our data suggest that the assessment of the number and extent of SCs may be somewhat subjective, as the interrater agreement was only moderate. Also, it seems that the assessment of PAs adds little to the information provided by the assessment of SC and AIS, so in future studies it might be convenient to simplify the score by eliminating this dimension.
In the future, as the availability of ultrasound equipment and the training of paediatricians in ultrasound grow, the use of this technique in the management of respiratory diseases may become routine. However, to reach the full potential of lung ultrasound, systematic and validated assessment protocols need to be developed. In our opinion, future studies should focus on detecting which findings are most useful in predicting the course of AB. This would require the inclusion of enough patients that progress to severe forms and need mechanical ventilation, so multicentre studies would be preferable to ensure adequate recruitment.
ConclusionsWe found a moderate correlation between the clinical severity scores and the degree of lung involvement evinced by early ultrasound examination in infants with mild-to-moderate AB. The most useful sonographic findings were those concerning the degree of AIS and SC. Lung ultrasound findings may be associated with clinical course and be useful to identify patients at risk of progression to severe disease.
FundingXIX Clinical and Epidemiological Research Grant Competition—2016, Fundación Ernesto Sánchez Villares, Sociedad de Pediatría de Asturias, Cantabria y Castilla y León. Project 02/2016: Utilidad de la ecografía pulmonar en la valoración de la gravedad y pronóstico de la bronquiolitis aguda. Principal investigator: Elia Zoido Garrote. Grant of 3000 euro.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Zoido Garrote E, García Aparicio C, Camila Torrez Villarroel C, Pedro Vega García A, Muñiz Fontán M, Oulego Erroz I. Utilidad de la ecografía pulmonar precoz en bronquiolitis aguda leve-moderada: estudio piloto. An Pediatr (Barc). 2019;90:10–18.