In June 2023, the working groups of the Spanish societies of paediatric pulmonology and infectious diseases (Sociedad Española de Neumología Pediátrica and Sociedad Española de Infectología Pediátrica) published the Update on the Diagnosis and Treatment of Tuberculosis.1 This document insists on the importance of adequate adherence to tuberculosis (TB) treatment, although it also highlighted that the formulations of first-line oral TB drugs currently marketed in Spain were not suitable for young children. In fact, only one oral solution of rifampicin is available. This is a problem shared by all countries in the European Union that forces paediatricians to resort to solutions that are not contemplated in the summary of product characteristics, such as crushing tablets, mixing them with food or using combinations of fixed-dose formulations developed for use in adults.2
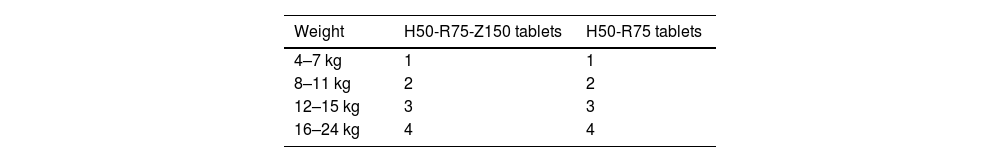
Since August 2023, after a long process of marketing authorization and importing with the collaboration of the Agencia Española de Medicamentos y Productos Sanitarios (AEMPS, Spanish Agency of Medicines and Medical Devices), dispersible fixed-dose combination formulations are available in tablet form for isoniazid (50 mg)-rifampicin (75 mg)-pyrazinamide (150 mg; HRZ) and isoniazid (50 mg)-rifampicin (75 mg; HR).3 Dispersible tablets are also available for monotherapy with isoniazid (100 mg) and ethambutol (100 mg). These formulations readily dissolve in water, have pleasant organoleptic properties and are administered in doses based on body weight ranges (Table 1), according to the recommendations for the dose per kg of body weight in young children. They also have a shelf life of 2 years and a reasonable price. They are useful for treatment of TB disease (HRZ tablets ± ethambutol tablets in the intensive phase; HR tablets in the maintenance phase), TB infection (HR tablets for 3 months or isoniazid as monotherapy for 6–9 months), and for primary chemoprophylaxis in young children after contact with an adult with active disease (isoniazid as monotherapy). Once dissolved in water, they must be administered immediately, although these tablets can also be taken whole. Although they were developed with young children in mind, in rare cases they may be useful for older patients with swallowing problems or when medication needs to be administered via a feeding tube.
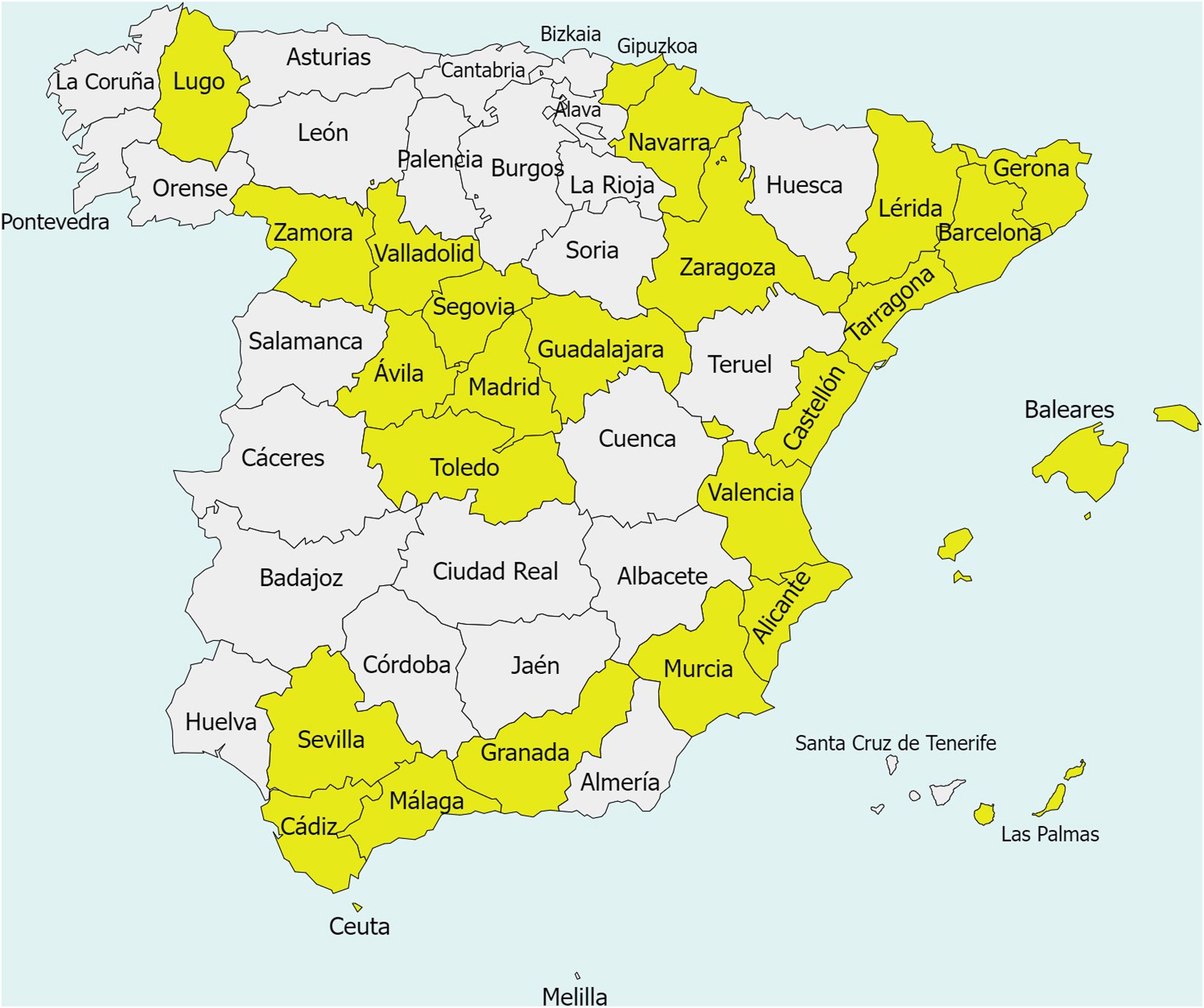
Since they became available in 2015, dispersible tablets have made possible the treatment of more than one million of children with TB in over 120 low-income countries worldwide. In Spain, any paediatrician can order them through a hospital pharmacy as a foreign drug after presenting an individual clinical report through the “Medication in Special Situations” procedure of the AEMPS.4 As of April 2024, 8 months after the first import, 25 out of 50 Spanish provinces (50%) and the autonomous city of Ceuta have requested at least one of the dispersible tables for treatment of at least 1 patient (Fig. 1). We do not know why half of the country has yet to use them. Our aim in writing this letter is to spread the news about these novel formulations to all paediatricians in Spain and highlight how they can facilitate the treatment of TB in paediatric patients, so that the maximum possible number of children can benefit from them.
This study was partially funded by the Instituto de Salud Carlos III, Ministry of Economy and Competitiveness of Spain (PI22/00766) and a grant of the Sociedad Española de Neumología y Cirugía Torácica (169-2022).
Conflicts of interestThe authors have no conflicts of interest to declare.