Over the last few years, growing knowledge of the pathogenesis of mastocytosis has allowed advances in the diagnosis, treatment and outcomes of these patients.1 Patients with mastocytosis and D816V variants in the KIT gene can benefit from treatment with midostaurin, an oral multi-targeted tyrosine kinase inhibitor (TKI).2 Variants other than KIT D816V are more frequent in children with mastocytosis, and these patients have been found to respond to other TKIs such as imatinib, nilotinib and masitinib.1 Paediatric patients with severe mastocytosis refractory to conventional therapy have been successfully treated with imatinib.1–5
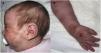
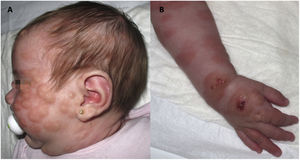
We present the case of a girl with diffuse cutaneous mastocytosis (DCM) who had onset in the first month of life with large plaques and bullous lesions on the face and body (Fig. 1) associated with flushing, abdominal pain and diarrhoea. A skin biopsy confirmed the diagnosis. Tryptase levels were elevated in the early months of life. The patient exhibited a limited response to treatment with steroids, antihistamines, cromoglycate and ketotifen. At age 4 years, her condition worsened, with development of frequent headaches and episodes of hypotension. She was treated with psoralen plus ultraviolet A (PUVA) phototherapy twice a week, which achieved an improvement in her symptoms and cutaneous lesions initially but became decreasingly effective over 10 months of treatment.
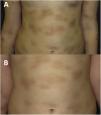
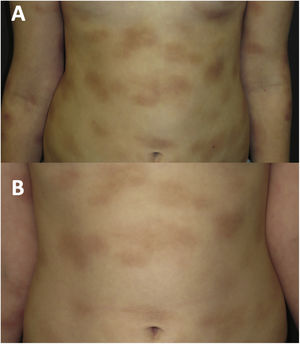
At age 8 years (Fig. 2A), the symptoms associated with heat, exercise and emotional triggers worsened. The tryptase levels were normal. A new skin biopsy revealed an infiltrate of mast cells with a granular cytoplasm that were c-KIT+, tryptase+, CD30+ and CD25−. A bone marrow biopsy and examination was performed, revealing normal cell counts and morphology. The allele-specific oligonucleotide real-time quantitative PCR (ASOqPCR) assay (TaqMan) did not detect the D816V variant in the KIT gene. However, fluorescence-activated cell sorting (FACS) isolated 0.0012% of mast cells with a c-KIT+, tryptase+, CD25− and CD30− immunophenotype. Sanger sequencing of genetic material from FACS-purified bone marrow mast cells did not find the D816V variant, but rather the M541L polymorphism in the KIT gene. Based on this finding, treatment with imatinib 100mg per day was initiated combined with the ongoing treatment with cromoglycate 200mg twice a day and ketotifen 1mg twice a day, with addition of oral antihistamines and steroids during mast cell flares.
During the 4 years of follow-up, the response to treatment has been satisfactory, with good tolerance of imatinib, an attenuation of infiltration in the cutaneous lesions (Fig. 2B) and improvement in systemic symptoms. Occasional episodes of dizziness have resolved with oral dexchlorpheniramine maleate. The patient’s growth has been normal, no adverse events have been detected, and laboratory test results and tryptase levels have remained normal.
Activating mutations of the KIT receptor tyrosine kinase have been reported in different types of cancer and in diffuse cutaneous mastocytosis. Imatinib is an oral TKI approved for treatment of systemic mastocytosis in patients with KIT mutations outside exon 17, but is generally not effective for D816V-associated disease.2 The M541L polymorphism found in our patient has been associated with paediatric mastocytosis and a greater sensitivity to imatinib therapy.3
The adverse events associated with the use of imatinib include nausea, vomiting, diarrhoea, transaminase elevation, cardiomyopathy, anaemia, thrombocytopenia, granulocytopenia, oedema, skin rash and decreased growth velocity in children.4,5
Although there is limited experience in the use of imatinib in children with mastocytosis, it could be an alternative option for patients with DCM and severe symptoms refractory to conventional therapies.
We thank the patient and the family who collaborated in the publication of this case, thus contributing to furthering the knowledge of this condition.
We also thank Dr Victor J. Asensio and Dr María Carmen Vidal of the Department of Genetics for their comments on the manuscript and Dr Ana Bauzá for providing the photographs of the patient in the early months of life.