The aim of this study was to evaluate the accuracy of imaging tests (prenatal ultrasound [US] and postnatal computed tomography [CT]) in comparison to histology for diagnosis of congenital lung malformations (CLMs).
Material and methodsRetrospective study of patients with a prenatal diagnosis of CLM whose postnatal follow-up included thoracic CT scan and histological examination of the lesion. We collected data on demographic variables, gestational age at diagnosis, US findings and the history of multiple gestation. We used the kappa coefficient to determine the level of agreement between the findings of prenatal US and postnatal tests (CT and histology).We analysed paired data on the size of the lesion, its location and the presence or absence of systemic arterial vascularization.
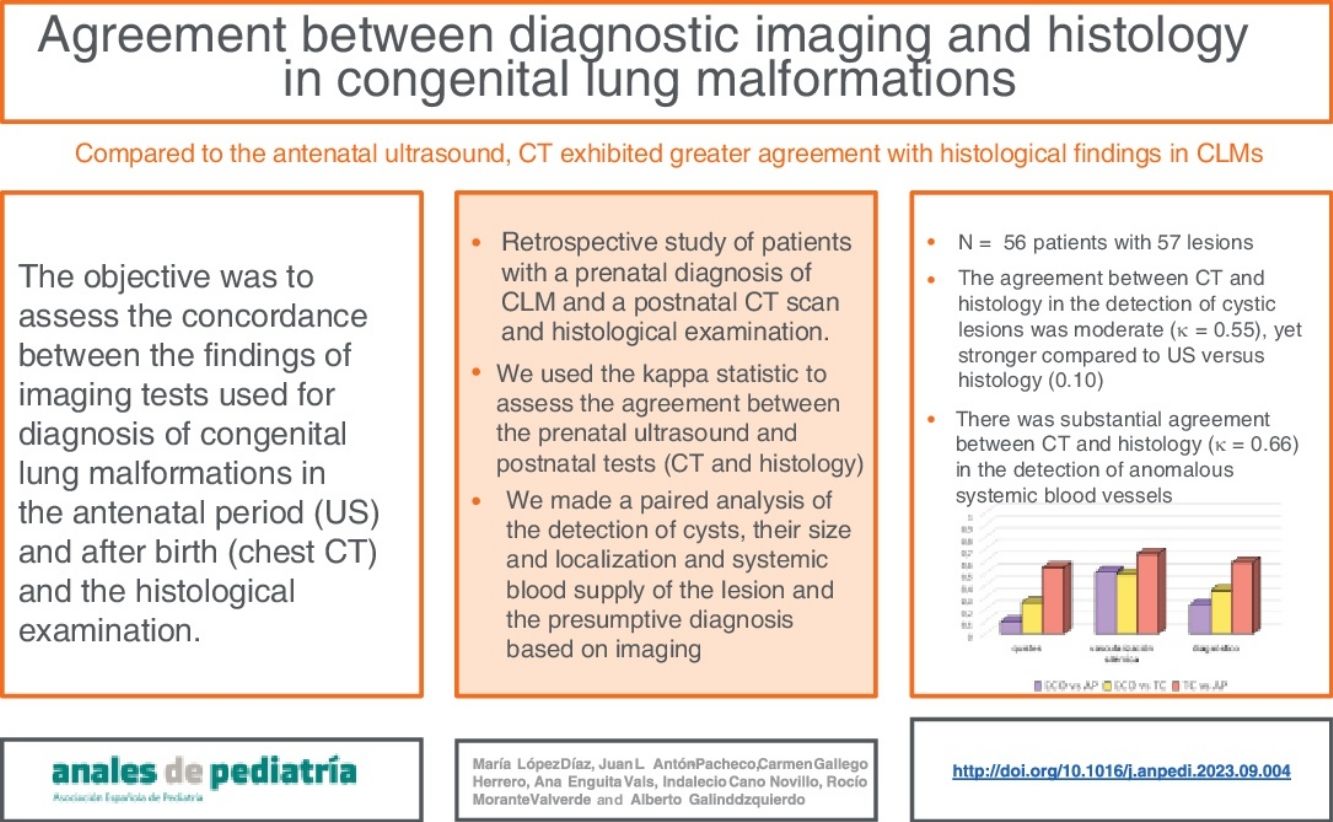
ResultsThe sample included 56 patients with 57 lesions. The mean gestational age at diagnosis was 22.42 weeks (SD, 3.94) and 57% were male. Malformations most frequently involved the left lung and the lower lobes. The agreement between CT and histology in the detection of cystic lesions was moderate (κ = 0.55) but stronger compared to the agreement between US and histology (κ = 0.10). The agreement between CT and histology was substantial (κ = 0.66) in the detection of systemic vascularization of the lesion and stronger compared to the agreement between US and histology. Both imaging methods were highly accurate in the identification of the location of the pulmonary lesions.
Conclusionspostnatal CT offers a substantial concordance with histological findings, especially in the detection of systemic vascularization, and an accurate prediction of the anatomy of the lesion.
El objetivo fue evaluar la concordancia entre las pruebas de imagen, ecografía prenatal y TC postnatal, empleadas en el diagnóstico de malformaciones pulmonares congénitas (MPC) y el estudio anatomopatológico (AP).
Material y métodosEstudio retrospectivo de pacientes diagnosticados prenatalmente de MPC en los que se realizó seguimiento postnatal incluyendo una TC y un estudio AP de la lesión. Las variables estudiadas incluyeron: datos demográficos, edad gestacional al diagnóstico, hallazgos ecográficos, y existencia de gestación múltiple. Utilizamos el coeficiente estadístico Kappa para establecer la concordancia entre la ecografía y las pruebas postnatales (TC y AP). Se analizaron de forma pareada la presencia de lesiones, localización, tipo y tamaño, y la presencia de vascularización sistémica.
ResultadosSe incluyeron 56 pacientes con 57 lesiones. La edad gestacional media al diagnóstico fue 22,42 ± 3,94 semanas y el 57% fueron varones. El pulmón izquierdo y los lóbulos inferiores fueron los más afectados. La concordancia entre TC y AP en la detección de lesiones quísticas fue moderada (Kappa = 0,55) pero más relevante que la detectada entre ECO y AP (Kappa = 0,10), siendo discreta entre ambas pruebas de imagen. La concordancia TC /AP fue sustancial (Kappa = 0,66) en la detección de vascularización sistémica de la lesión y superior a la determinada entre ecografía y AP. Ambas pruebas de imagen demostraron una precisión muy buena en la identificación de la localización de las lesiones.
ConclusionesLa TC postnatal ofrece una concordancia sustancial con el estudio histológico, especialmente en la detección de vascularización, y nos aporta datos predecibles sobre la anatomía de la lesión.
Congenital lung malformations (CLMs) are a heterogeneous group of abnormalities of pulmonary development with an incidence ranging 1 per 2500 to 1 per 27 400 live births.1–4 They include congenital pulmonary airway malformations (CPAMs), intralobar pulmonary sequestration (ILPS) and extralobar pulmonary sequestration (ELPS), congenital lobar overinflation (CLH), bronchogenic cyst and bronchial atresia.1,2,5–8 The most frequent anomaly is CPAM, which accounts for approximately 95% of all CLMs, with an incidence estimated at 1–5 per 10 000 births.9,10 The clinical presentation of CPAMs varies widely, ranging from asymptomatic small pulmonary lesions to large cysts that can cause severe respiratory distress from birth.11
Due to the widespread implementation of antenatal ultrasound screening, the incidence of CLMs has increased significantly in the past two decades, chiefly on account of the early detection of asymptomatic cases.8 At the same time, controversy has emerged regarding the optimal approach to the management of patients with subclinical malformations. On one hand, there is the option of elective surgery (before age 2 years), supported by the increased risk of respiratory infection, pneumothorax and, in some cases, malignant degeneration, in addition to a faster and better recovery after surgery.12,13 On the other hand, there is some evidence in support of conservative management with long-term clinical and imaging follow-up, justified by the current lack of knowledge on the natural history of these malformations and their potential for regression, which has been documented in up to 15% of cases.14 To this, we must add the risks associated with a major surgery procedure involving thoracotomy or thoracoscopy with lung resection.11–18 In this context, an early and accurate diagnosis of CLMs is essential to determine the most appropriate perinatal management of these patients and offer families detailed information based on the current evidence.8 Sonography is the diagnostic method of choice for the antenatal detection of CLMs as it is a safe, reproducible, widely available and inexpensive technique. In the case of diagnostic uncertainty, it may be followed by foetal magnetic resonance imaging.6,8,10,12,19 If a CLM is detected antenatally, early performance of a chest computed tomography (CT) scan in the first month of life is recommended in the case of large or bilateral lesions or multifocal cysts or if the neonate develops pneumothorax. Generally, this test is deferred until 6–8 months post birth in asymptomatic patients who do not meet any of the aforementioned criteria.2,6,12
The aim of our study was to assess the concordance between the findings of imaging tests used for diagnosis of CLMs in the antenatal period (ultrasound scan) and after birth (chest CT scan) and the histological examination. In doing so, we sought to establish the accuracy of these imaging tests in the characterization of lesions consistent with MCP using the histological examination as reference, which is considered the gold standard for diagnosis of MCPs. Our hypothesis was that the findings of the antenatal ultrasound in CLMs correspond reliably with those of the postnatal chest CT scan, which would make the latter test unnecessary in asymptomatic newborns and infants.
Material and methodsStudy designWe conducted a retrospective study through the analysis of data on patients given an antenatal diagnosis of CLM between June 2005 and July 2020 in a tertiary care referral hospital, obtained from a prospective database. We retrieved and handled the data through the Research Electronic Data Capture (REDCap) system of our hospital. The REDCap is a secure online application developed to support data collection for research studies. This tool is very useful, as it provides1: an intuitive interface for entering validated data,2 audit tracking software to track data handling and exporting,3 automated export procedures to transfer data seamlessly to commonly used statistical software packages and4 routines to import data from outside sources.20 The analysis included patients with an antenatal diagnosis of CLM that had remained in follow-up after birth, including performance of a CT scan. We excluded patients with an antenatal diagnosis of CLM who were not followed up after birth or did not undergo a CT scan. The study was approved by the Research Ethics Committee of our hospital (code 21/393) and we obtained informed consent for all patients from their parents to perform surgery and obtain video and photographic content.
Diagnostic protocol and data collectionAntenatal screening was performed with high-performance ultrasound machines: Siemens Antares; General Electric Voluson E8 and E10 Expert ultrasound machine. The postnatal diagnosis was conducted by means of helical CT scan with intravenous contrast under general anaesthesia. The equipment used for CT until 2010 was the Toshiba Xpress/ex Aspire scanner with one detector at 120 mA and 80 Kv with external protection elements. From then on, it was a Brilliance CT 64-slice scanner (Philips Medical Systems) with 64 detectors, with the tube voltage set at 100 mA, automated current modulation technique without external protection devices.
All patients in the sample received an antenatal diagnosis of CLM by a specialist in foetal medicine in the department of gynaecology and obstetrics of our hospital. The data were collected from the radiology reports. The prenatal variables under study were: demographic characteristics, gestational age at diagnosis, sonographic findings and type of pregnancy (singleton or multiple). Patients with an antenatal diagnosis of CLM were followed up postnatally by specialists in the department of paediatric surgery. All of them underwent a chest CT scan, usually between 3 and 8 months post birth. In patients with lesions suggestive of malignant disease in the antenatal ultrasound (CPAMs type IV or pleuropulmonary blastoma, according to the Stocker classification system),9 clinically significant respiratory symptoms or pneumothorax in the neonatal period, the CT scan was performed early in the first month of life. The images were reviewed during the data collection period by a radiologist expert on CLMs. The most common findings of both imaging tests (antenatal ultrasound and postnatal CT) were: presence of cysts, size and location of cysts, anomalous systemic vessels and diagnostic impression obtained with each technique.
In operated patients, the initial histological examination of the pulmonary lesions was carried out by different pathologists in the hospital. However, for the purpose of this study, a pathologist with expertise on the subject reviewed all the slides anew. We considered the histological examination the gold standard for the definitive diagnosis of CLMs. Patients who did not undergo surgery, and therefore lacking a histological examination, were excluded from the final analysis.
We classified lung malformations based on the most recent update of the Stocker classification system.9
Statistical analysisThe aim of the analysis was to determine whether the same result could be obtained with different diagnostic tests. To do so, we used the kappa statistic, which allowed us to measure the level of agreement between the antenatal technique (ultrasound) and postnatal techniques (CT and histology). We assessed the detection of cysts, their location, detection of anomalous systemic vessels and the presumptive diagnosis. We did this analysis by pairs: ultrasound vs CT, ultrasound vs histology, CT vs histology. The analysis of concordance was based on nominal qualitative variables, and we used the Cohen kappa in the case of dichotomous qualitative variables and the Cohen weighted kappa for non-dichotomous qualitative variables. We used the following intervals in the interpretation of the kappa statistic:
- •
Kappa 0.00−0.20: none to slight agreement.
- •
Kappa 0.21−0.40: fair agreement.
- •
Kappa 0.41−0.60: moderate agreement.
- •
Kappa 0.61−0.80: substantial agreement.
- •
Kappa 0.81–1.00: almost perfect agreement.
We considered results significant if the P value was less than 0.05 (95% confidence interval [CI]). All the analyses were performed with the SAS statistical software suit, version 9.4 for Windows.
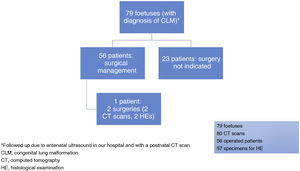
ResultsCongenital lung malformations were detected in a total of 79 foetuses, of who 56 (70.8%) underwent surgery postnatally; surgery was not indicated in the remaining patients (Fig. 1). Fifty-seven surgical specimens were obtained for histological examination, as one patient had a bilateral CLM that required 2 asynchronous operations. The mean gestational age at diagnosis was 22.42 weeks (standard deviation, 3.94) and 57% (45/79) of the patients were male. Macrocystic lesions, measuring more than 5 mm, were the most frequent type of lesion in this case series (45.5%). As regards location, based on the antenatal ultrasound, the left lung (54.4%; 43/79) and the inferior lobes (74.6%; 59/79) were involved most frequently. Table 1 summarizes the demographic characteristics of the patients and the antenatal ultrasound findings.
Demographic and prenatal data.
| Male sex | 45 |
|---|---|
| Detection in 2nd trimester | 65a |
| Detection in 3rd trimester | 13a |
| Gestational age at birth (median) | 39 |
| Birthweight in kg (median) | 3.2 |
| Multiple gestation | 4 |
| Prenatal anomalies | 13 |
| Mediastinal shift | 35 |
| Pleural effusion | 5 |
| Thoracoamniotic shunting | 3 |
| Hydrops fetalis | 1 |
| Polyhydramnios | 3 |
| Amniodrainage | 1 |
| Macrocystic lesions | 36 |
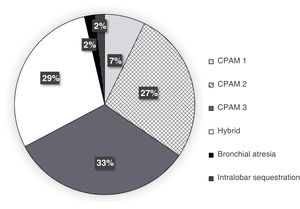
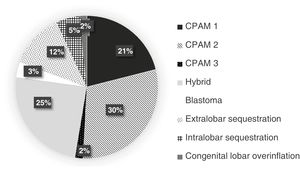
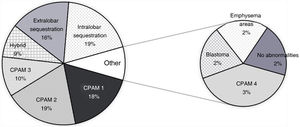
Figs. 2 and 4 present the distribution and frequency of each type of lesion based on the diagnostic technique used. Overall, most of the detected malformations were CPAMs, both in ultrasound and CT scans. Hybrid CPAM/sequestration lesions (25%), extralobar or intralobar pulmonary sequestration (12% and 5%, respectively) and congenital lobar overinflation (2%) were found less frequently (Figs. 2 and 3).
We assessed the agreement between the 3 diagnostic tests based on the following variables:
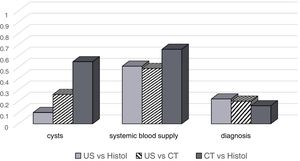
CystsThe analysis of agreement in the detection of cystic lesions found poor agreement (κ = 0.10) between antenatal sonography and histology. On the other hand, the agreement between CT and histology was moderate (κ = 0.55). The agreement between the two imaging methods, sonography and CT, was fair (κ = 0.26) (Fig. 5). The sensitivity and specificity of sonography for the detection of cysts were 100% and 9%, respectively. When it came to CT, the sensitivity was 100% and the specificity 50%.
LocalizationThe agreement between sonography and CT in regard to the laterality of the lesions was very good. The Cohen kappa was 0.92 for localization in the right lung (95% CI, 0.8314–1.0), and 0.96 for localization in the left lung (95% CI, 0.8947–1.0) (P <. 0001). The agreement between sonography and CT in the detection of bilateral malformations (n = 2) was perfect (κ, 1). In a more detailed analysis by lobe, for the right lung we found a kappa of 0.34 for the superior lobe, of 0.03 for the middle lobe and of 0.89, the highest agreement, for the inferior lobe. When it came to the left lung, we found a kappa of 0.50 for the superior lobe and stronger agreement, with a kappa of 0.74, for the inferior lobe.
Systemic vascular connectionThe agreement between sonography and histology and between sonography and CT in the detection of anomalous systemic vessels was moderate (κ = 0.51 and 0.49, respectively). The agreement was stronger between CT and histology (κ = 0.66) (Fig. 5). The sensitivity of the antenatal ultrasound for detection of systemic vascular connections was 60%, and the specificity was 89%. In the case of CT, the sensitivity was 85% and the specificity 84%.
Presumptive diagnosis based on imaging findingsGiven the disparities in the number of potential diagnoses that can be made depending on the technique used (6 with sonography, 8 with CT and 10 with histology; Table 2), we found poor agreement between the presumptive diagnosis made by CT and the definitive diagnosis based on the histological findings (Kappa 0.16). In the comparisons of sonography vs histology and sonography vs CT, the agreement was fair, with kappa values of 0.22 and 0.20, respectively (Fig. 5).
Distribution of diagnoses based on the diagnostic method.
| Histology | Sonography | Computed tomography |
|---|---|---|
| 10 CPAM type 1 | 1 HL, 2 CPAM type 1, 5 CPAM type 2, 2 CPAM type 3 | 5 CPAM type 2, 5 CPAM type 1 |
| 1 blastoma | CPAM type 3 | CPAM blastoma |
| 11 CPAM type 2 | 1 HL, 1 CPAM type 1, 2 CPAM type 2, 6 CPAM type 3, 1 ND | 1 HL, 3 CPAM type 1, 7 CPAM type 2 |
| 6 CPAM type 3 | 1 HL, 3 CPAMs type 2, 2 CPAM type 3 | 2 HL, 1 CPAM type 1, 1 CPAM type 2, 1 CPAM type 3, 1 CPAM/blastoma |
| 2 CPAM type 4 | 1 CPAM type 1, 1 CPAM type 2 | 2 CPAM type 1 |
| 5 HL | 1 HL, 1 CPAM type 2, 2 CPAM type 3, 1 ND | 4 HL, 1 CPAM type 2 |
| 9 ELPS | 7 HL, 1 ILPS, 1 CPAM type 3 | 7 ELPS, 2 ILPS |
| 11 ILPS | 4 HL, 3 CPAM type 2, 4 CPAM type 3 | 7 HL, 1 ILPS, 1 CPAM type 1, 2 CPAM type 2 |
| 1 No abn. | 1 HL | 1 CPAM type 2 |
| 1 area of emphysema | 1 bronchial atresia | 1 CLH |
CLH, congenital lobar overinflation; CPAM, congenital pulmonary airway malformation; ELPS, extralobar pulmonary sequestration; HL, hybrid lesion; ILPS, intralobar pulmonary sequestration; ND, no data available; No abn, no abnormalities.
We found, overall, slight agreement between the diagnosis made by imaging (antenatal ultrasound/postnatal CT) and histology. The agreement was stronger for the detection of systemic vascular connections, especially between CT and histology, and when the possible diagnoses were grouped. The use of the kappa statistic to assess the agreement between imaging tests and histological diagnosis is a novel contribution of this study. Unlike other statistical methods, the kappa statistic allows paired comparisons and offers more precise information regarding the reliability of each test for the variables under study. The use of this new approach may have contributed in part to the discrepancies between our findings and those of other authors.8,21
It is clear that it is difficult to make presumptive diagnoses using imaging methods but based on histological criteria For this reason, Farrugia et al.21 proposed a classification of CLMs based solely on anatomical criteria (nature of the lesion, arterial supply and venous drainage) aimed at reducing the discrepancies between radiological, intraoperative and histological findings. In our study, we followed a similar approach, since a too detailed breakdown of the study variables and potential histological diagnoses could have made the correlation between imaging and histological findings irrelevant. In addition to the type of lesion (cystic or solid) and its potential connection to the systemic circulation, we added other variables, such as the location of the malformation and the presumptive diagnosis. With this approach, we obtained a moderate correlation between CT and histology in the detection of cystic lesions (κ = 0.55) ad a much weaker correlation between sonography and histology (κ = 0.10). The agreement between the two imaging tests was fair for this variable (κ = 0.26). The agreement between CT and histology in the detection of anomalous systemic vessels connecting to the lesion was substantial (κ = 0.66) and, once again, stronger compared to the agreement observed between sonography and histology. Both imaging tests exhibited very high accuracy in identifying the location of the lesions. In the separate analysis by lobe, we found the strongest agreement for the inferior lobes, especially the right inferior lobe. However, the agreement between sonography and histology was only slight when we compared the presumptive diagnoses. The agreement was even lower between CT and histology (κ = 0.16), which may seem contradictory in light of the results concerning the type of lesion and its vascularization. We believe that the dispersion in the number of diagnoses, as many as 10 possible with histology compared to 8 with CT, may explain why the value of the statistic was that insignificant, even lower than the statistic for the comparison of sonography and histology, in which only 6 possible diagnoses were included in the analysis.
There is no question that imaging tests are essential in the detection and description of pulmonary lesions and in reaching a presumptive diagnosis. The diagnosis of CLMs with imaging techniques has improved significantly in recent years, allowing earlier detection, usually between weeks 18 and 22 of gestation.6,21 Most patients with an antenatal diagnosis have favourable outcomes over the perinatal period.15 However, the development of complications such as polyhydramnios, hydrops fetalis or mediastinal shift in 10% of cases and, on the other hand, the fact that up to 45% of the lesions are symptomatic after birth, make ultrasound screening during pregnancy indispensable, as it allows adequate planning of childbirth and perinatal care.21 In the sample under study, 7 patients (12.5%) underwent surgery due to the presence of symptoms (infection in 3 and pulmonary disease in 4).
Most of the pulmonary lesions detected by either antenatal sonography or postnatal CT were classified as CPAMs (Fig. 3), which was consistent with most studies in the previous literature.6,8,12 When it came to the histological diagnosis, the distribution found in our study was also very similar to the distribution reported in previous studies, with CPAMs as the most frequent type of malformation, followed by sequestration and hybrid malformations22 (Fig. 4). Congenital pulmonary airway malformations comprise a spectrum of anomalies ranging from cystic lesions of varying size to solid adenomatoid masses.9 There is evidence that lesions with large cysts (>2 cm) can undergo malignant transformation over time, and the potential transformation of Stocker type 4 lesions with large multiloculated cysts into pleuropulmonary blastoma (PPB) is particularly relevant.9 In this case series, 2 patients had features suggestive of PPB, confirmed by histology in one. Certain risk factors for PPB have been identified, such as the association of pneumothorax with bilateral large multiloculated cysts.1 The very good agreement found in our study between the antenatal ultrasound and the postnatal CT in the identification of the localization and bilaterality of lesions is particularly relevant in this regard. In addition, we observed a predominance of CLMs in the left lung and the inferior lobes, consistent with the recent literature.6,15 This could be explained by the more common involvement of the inferior lobes in some of the most frequent diseases, such as CPAMs and bronchopulmonary sequestration (Fig. 6).
There are some limitations to this study, such as its retrospective design and, focusing on a given cohort, a relatively small sample size. On the other hand, although it was not possible to review the antenatal ultrasound images at the time of the study, it was possible to review the postnatal CT scans (reviewed by an expert radiologist blinded to the histological findings) and the histological findings (reviewed by a pathologist with expertise in the microscopic examination of surgical specimens).
In conclusion, the level of agreement between the antenatal ultrasound and the histological examination is far from good enough to use the former technique as the sole method to guide therapeutic decision-making in the perinatal and postnatal period in patients with a diagnosis of CLM. On the other hand, the postnatal CT scan exhibited substantial agreement with the histological examination, especially in the detection of systemic vascular connections, and provided information useful to predict the anatomy and localization of the lesions. The information contributed by the CT scan may be very useful for planning any surgery, be it elective or urgent, that needs to be performed in patients with symptomatic malformations or lesions suspected to be malignant.12,23
Conflict of interestsThe authors have no conflicts of interest to declare.
We thank Carmen Romero Ferreiro for her collaboration in the statistical analysis and Dr María Enery Gómez Montes for providing us the photographs.