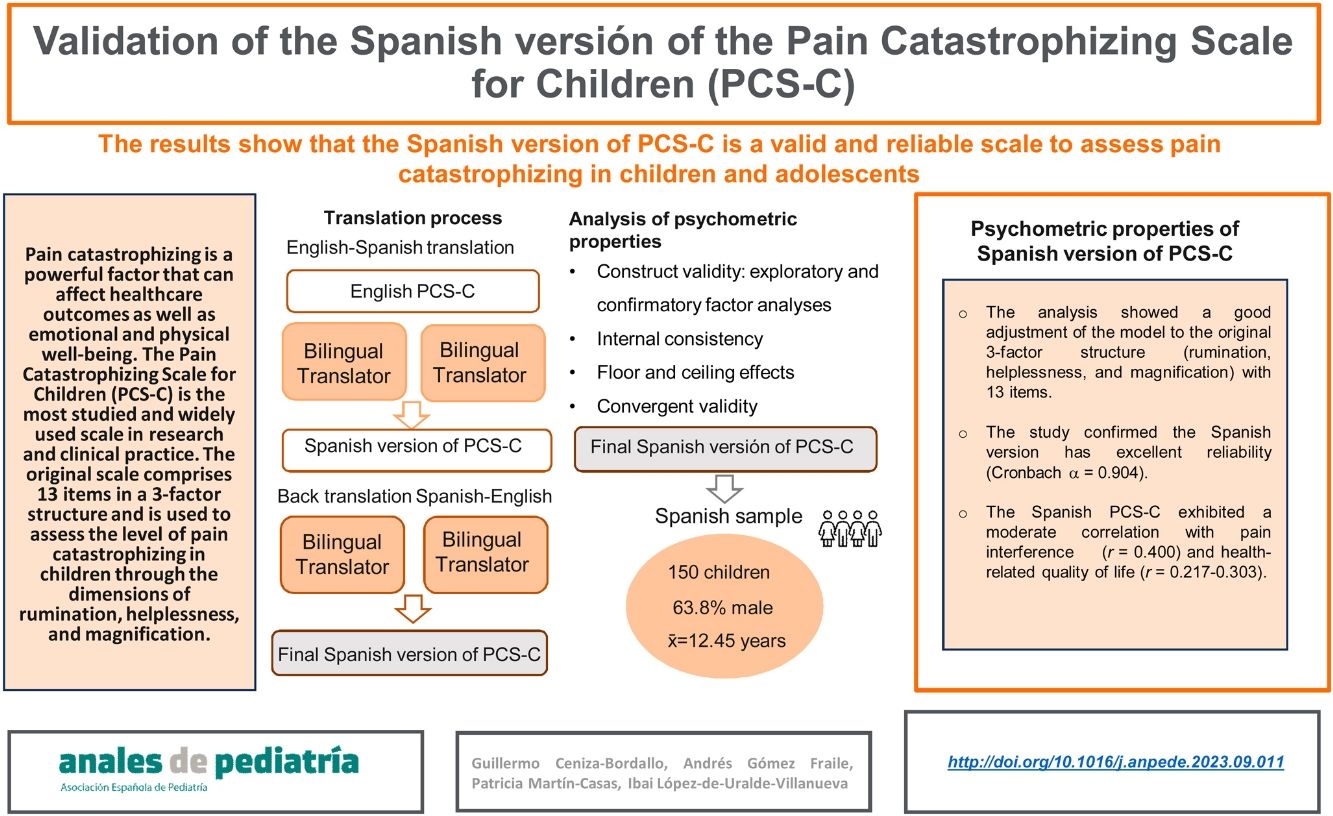
Pain catastrophizing is a powerful factor that can affect health care outcomes as well as emotional and physical well-being. The Pain Catastrophizing Scale for Children (PCS-C) is widely used, but it is not validated in Spanish. The aim of the study was to translate the PCS-C to Spanish and assess the validity and reliability of the translated version.
Patients and methodsThis study was carried out in two phases: (a) instrument translation (via a translation-back-translation process) and (b) psychometric analysis (construct validity: exploratory and confirmatory factor analysis, internal consistency, floor and ceiling effects and convergent validity). It had a cross-sectional design and was conducted on a sample of children aged 8–18 years was selected by convenience in a paediatric hospital. The study followed the STARD checklist.
ResultsThe sample included 150 children and adolescents (mean age, 12.45 years; 63.8% male) and their parents. The exploratory and the confirmatory analysis showed a good adjustment of the model to the original 3-model structure with 13 items. The internal consistency of the scale was excellent (Cronbach α, 0.904), and no floor or ceiling effects were detected. In the convergent validity analysis, the Spanish version of the PCS-C showed a moderate correlation with pain interference (r=0.400) and with health-related quality of life (r=0.217–0.303).
ConclusionsThese results show that the Spanish version of the PCS-C is a valid and reliable scale to assess pain catastrophizing in children and adolescents.
El catastrofismo relacionado con el dolor es un factor influyente en el pronóstico del tratamiento, así como en el bienestar emocional y físico. La escala pediátrica Pain Catastrophizing Scale for Children (PCS-C) es ampliamente utilizada, pero no está validada en español. Este estudio tuvo como objetivo traducir la PCS-C al español y evaluar su validez y fiabilidad.
Pacientes y métodosEste estudio se llevó a cabo en dos fases: a) traducción del instrumento (mediante un proceso de traducción directa e inversa) y b) análisis psicométrico (validez de constructo: análisis factorial exploratorio y confirmatorio, consistencia interna, efectos suelo y techo, y validez convergente) a través de un estudio transversal con una muestra seleccionada por conveniencia, de un hospital pediátrico compuesta por niños de 8 a 18 años. Este estudio siguió la lista de verificación STARD.
ResultadosEn el estudio se incluyó a 150 niños y adolescentes (edad media=12,45; 63,8% varones) y sus padres. El análisis exploratorio y posteriormente el análisis confirmatorio mostraron un buen ajuste del modelo a la estructura original de tres modelos con 13 ítems. La consistencia interna de la escala resultó excelente (α de Cronbach=0,904) y no se detectaron efectos techo ni suelo. En cuanto al análisis de validez convergente, la PCS-C en español mostró una correlación moderada con la interferencia del dolor (r=0,400) y con la calidad de vida relacionada con la salud (r = 0,217–0,303).
ConclusionesEstos resultados demuestran que la versión en español de la PCS-C es una escala válida y fiable para evaluar el catastrofismo relacionado con el dolor en niños y adolescentes.
In children, pain catastrophizing is a psychological factor associated with several essential components in pain research and can have an impact on treatment outcomes as well as emotional and physical well-being.1–4 It has been defined as “an exaggerated negative ‘mental set’ brought to bear during actual or anticipated pain experience”5 and is of great importance due to its impact on functioning outcomes in models of paediatric chronic pain.3 Specifically, it is an important factor in the paediatric fear-avoidance model of pain, according to which children with catastrophic thinking perceive pain as a threat and experience hypervigilance and maladaptive behaviors in association with pain.6,7 This cycle of functional and emotional disability is frequent in patients with pain, especially in paediatric patients with chronic pain.8 Similarly, pain catastrophizing plays an essential role in the transition from acute pain to chronic pain.6,7
Several instruments are available for the assessment of pain catastrophizing, among which the Pain Catastrophizing Scale-Child version (PCS-C) is the most studied and widely used in research and clinical practice.9–15 The original scale comprises 13 items in a 3-factor structure and is used to assess the level of pain catastrophizing in children through the dimensions of rumination, helplessness, and magnification.9 Studies have been conducted to assess its psychometric properties and improve its usability and comprehensibility, prompting consideration of the possibility of modifying the original scale. This scale is used worldwide, as there are versions in various languages, but there is no Spanish version yet. The objectives of this study were to translate the PCS-C to Spanish and to evaluate its psychometric properties in a paediatric sample in Spain.
MethodsDesignThis study was carried out in two phases: phase I involved the translation of the instrument, and phase II the validity and reliability assessment.
Phase IInstrument: Pain catastrophizing was assessed by means of the PCS-C. This is a 13-item self-report scale developed by Crombez et al.9 to assess catastrophic beliefs associated with the experience of pain in children. The PCS-C assesses 3 dimensions (magnification, rumination, and helplessness) with a 5-point response scale (“not at all”, “mildly”, “moderately”, “severely” and “extremely”). Higher scores indicate stronger catastrophic beliefs about pain (the possible score ranges from 0 to 52). The English and Catalan versions of the PCS-C have proven to be reliable and valid in community and clinical samples of children and adolescents.9,13,14
Translation process: The PCS-C scale was translated from English, which was the original language of the scale,9 into Spanish following the translation and back-translation process. Two bilingual pain research experts translated the scale from English to Spanish independently. A professional bilingual translator resolved the discrepancies between the 2 versions of the pain researchers. Later, two bilingual natives back translated the Spanish version into English, and a professional bilingual translator compared this version with the initial version proposed by Crombez et al.9 The response options for intensity rating (0=not at all, 1=mildly, 2=moderately, 3=severely and 4=extremely) were replaced by frequency options (0=never, 1=rarely, 2=sometimes, 3=often and 4=always) because it was observed that this alternative improved comprehension in Spanish, as had been the case in the Catalan version.13 The Spanish version of the PCS-C can be found in Supplementary Document I.
Phase IIEthical Considerations: The study was approved by the ethics committee of the hospital and adhered to the principles of the Declaration of Helsinki Declaration. It was conducted following the Standards for Reporting Diagnostic Accuracy Studies (STARD).17
ParticipantsThe convenience sample included children and adolescents. The inclusion criteria were (a) ability to read and write in Spanish by both child and parents; and (b) age between 8 and 18 years (c) paediatric surgical patient classified as American Society of Anesthesiologists (ASA)16 Physical Status 3 or lower, which excluded patients with severe illnesses or those requiring urgent surgical interventions. Children with an intellectual disability that could interfere with the implementation of the study protocol, children undergoing urgent surgery and oncological patients were excluded.
ProtocolWe extended an invitation to participate in the study to children scheduled for surgery and their parents upon hospital admission. If the patients were interested in participating, a 45-minute interview was held with the parents, in which they were informed in detail about the study, completed the informed consent and filled out the print questionnaires. In order to participate, in addition to parental consent, children aged less than 12 years had to provide their assent, and adolescent participants their consent. Then, the children were interviewed apart from their parents so that their presence would not influence the response to the questionnaires (also in print format); this interview also lasted 30min. All interviews took place in the department of surgery of the hospital.
Outcome variablesTo carry out the validation, we assessed demographic characteristics and the following variables: the child’s previous surgeries, number of medical visits in the last year, pain intensity, assessed by the child (Faces Pain Scale-Revised [FPS-R]18) and by the parents (Parent’s Postoperative Pain Measure [PPPM]19), health-related quality of (Pediatric Quality of Life Inventory [PedsQL] version 4.0 Generic Core Scale20), functional disability (visual numeric rating scale [NRS]18) and pain interference (Patient-Reported Outcomes Measurement Information System [PROMIS] Pain Interference form21). Information on the characteristics of the scales and their psychometric properties can be found in Supplementary Document II.
Statistical analysisThe data were analysed with the SPSS version 21 (IBM SPSS Statistics) and the Mplus version 7.11 statistical software packages. The level of statistical significance was set at 5% (P<.05).
Construct validityA 2-step process was used to examine the validity of the construct: (1) exploratory factor analysis (EFA) to identify the optimal factor structure and (2) confirmatory factor analysis (CFA) to confirm the theoretical factor structure. Based on the criteria established by several authors, we estimated that a sample size of at least 100 children was needed to perform an adequate EFA.22,23 To confirm the theoretical factor structure, we increased the sample size by 50 children because in cases in which the data are well conditioned, samples of 50 participants can generate reliable results.22 Thus, the CFA was performed with a total of 150 observations.
We used the Bartlett test of sphericity and the Kaiser-Meyer-Olkin (KMO) to determine whether the Pearson correlation matrix was suitable for factor analysis.23 Following recommendations, we determined the optimal number of factors based on the Kaiser criterion (eigenvalue ≥ 1; scree plot).24 In the EFA, factors were extracted with the principal axis factoring with oblique rotation method. We established that for an item to be included in a factor, it needed to have a factor loading greater than 0.4.
The CFA was performed using the maximum likelihood with robust standard errors (MLR) estimation method. We used the following goodness of fit indices: chi squared (χ2), comparative fit index (CFI), Tucker Lewis index (TLI), standardized root mean square residual (SRMR) and root mean square error of approximation (RMSEA). We applied the Hu and Bentler criteria to determine whether the fit of the model was acceptable (TLI and CFI≥0.95, SRMR and RMSEA≤0.08).25 Furthermore, modification indices were calculated to identify local mis-specified areas of the model not sensitive to the overall goodness of fit indices previously mentioned.26
Reliability, floor and ceiling effectsWe assessed the reliability of the Spanish version of the PCS-C based on its internal consistency. The internal consistency would be considered acceptable if the Cronbach α was greater than 0.70.27 We defined floor or ceiling effect as at least 15% of patients achieving the minimum or maximum score, respectively.28
Convergent validityWe assessed convergent validity based on the Pearson correlation coefficient obtained in the comparison of the Spanish version of the PCS-C with the following clinical measures aimed at assessing pain, functional disability and quality of life in children: medical consultations in the last year; previous surgeries; FPS-R; PPPM; PROMIS Pediatric Pain Interference score; NRS for Functional Disability; PedsQL. A coefficient greater than 0.60 was considered indicative of a strong correlation; a coefficient of 0.30–0.60 of a moderate correlation and a coefficient of less than 0.30 of a weak correlation.28
ResultsThe study was conducted from June to September of 2020. We contacted a total of 180 school-aged children and their parents upon admission to the department of surgery of the children’s hospital of a tertiary care referral hospital. Thirty declined to participate in the study due to time concerns (17 participants), anxiety, stress or nerves (10 participants) and personal problems (3 participants). A total of 150 participants enrolled in the study, with a mean age of 12.45 years (standard deviation [SD], 2.64) (Table 1).
Anthropometric and sociodemographic characteristics of the children (N=150) and their parents/guardians.
| Mean±SD or n (%) | Range (min-max) | |
|---|---|---|
| Child’s age (years) | 12.45±2.64 | 8−18 |
| Child’s sex (male: female) | 96 (63.8%): 54 (36.2%) | |
| Child’s height (cm) | 154.57±16.74 | 100−198 |
| Child’s weight (kg) | 51.74±18.52 | 23−121 |
| Child’s BMI (kg/m2) | 21.11±4.84 | 13.9−39.1 |
| Child’s educational attainment | ||
| Primary education | 65 (43.3%) | |
| Secondary school/high school | 72 (48%) | |
| Vocational training | 13 (8.6%) | |
| Child’s previous surgeries | 0.94±1.96 | 0−18 |
| Medical consultations in the last year | ||
| 1 to 3 | 76 (50.6%) | |
| 4 to 6 | 47 (31.3%) | |
| 7 to 10 | 14 (9.3%) | |
| 11 to 14 | 8 (5.3%) | |
| 15 to 20 | 2 (1.3%) | |
| >20 | 3 (2%) | |
| Parent/guardian’s age (years) | 43.62±5.99 | 24−57 |
| Parent/guardian’s sex (male: female) | 23 (15,3%): 127 (84%) | |
| Parental educational level | ||
| Primary education | 17 (11.3%) | |
| Secondary education | 40 (26.6%) | |
| Professional training | 41 (27.3%) | |
| University education | 52 (34.6%) | |
| Family socioeconomic status | ||
| Low | 58 (38.6%) | |
| Medium | 89 (59.3%) | |
| High | 3 (2%) | |
BMI, body mass index; SD, standard deviation.
We calculated the Cronbach α for the entire scale (α=0.89) and for the adjusted item-total correlations (average item-total correlations=0.583) prior to the EFA. No items were removed, since all contributed substantially to the scale.
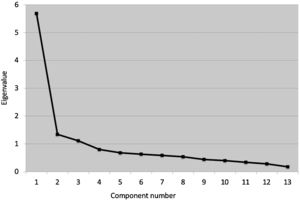
The KMO showed that the data was suitable for factor analysis (KMO=0.866), and the Barlett test of sphericity refused the identity matrix null hypothesis: χ278=592.41 (P<.001). According to these results, it was appropriate to proceed with the EFA. The Kaiser criterion indicated that the 3 factors should be retained (see Fig. 1). In addition, the goodness of fit indices showed that the 3-factor model fit the data well: CFI=0.980; TLI=0.963; SRMR=0.035; RMSEA=0.049; 95% CI: 0.001–0.088. Table 2 presents the factor loading of each of the items.
Exploratory factor analysis solution.
| Item | Helplessness | Magnification | Rumination |
|---|---|---|---|
| 1. Cuando tengo dolor, me preocupa constantemente si el dolor se irá. | .483* | ||
| When I am in pain, I worry all the time whether pain will end. | |||
| 2. Cuando tengo dolor, siento que no puedo seguir así mucho tiempo. | .462* | ||
| When I am in pain, I feel I can’t go on like this much longer. | |||
| 3. Cuando tengo dolor, es terrible y pienso que nunca va a mejorar. | .768* | ||
| When I am in pain, it’s terrible and I think it’s never going to get better. | |||
| 4. Cuando tengo dolor, es horrible y siento que puede conmigo. | .783* | ||
| When I am in pain, it’s awful and I feel it takes over me. | |||
| 5. Cuando tengo dolor, no puedo soportarlo más. | .507* | ||
| When I am in pain, I can’t stand it anymore. | |||
| 6. Cuando tengo dolor, tengo miedo de que empeore. | .489* | ||
| When I am in pain, I become afraid that the pain will get worse. | |||
| 7. Cuando tengo dolor, no dejo de pensar en otros hechos dolorosos. | .735* | ||
| When I am in pain, I keep thinking of other painful events. | |||
| 8. Cuando tengo dolor, quiero que el dolor desaparezca. | .722* | ||
| When I am in pain, I want the pain to go away. | |||
| 9. Cuando tengo dolor, no me puedo quitar el dolor de mi cabeza. | .861* | ||
| When I am in pain, I can’t keep it out of my mind. | |||
| 10. Cuando tengo dolor, no dejo de pensar en lo mucho que me duele. | .949* | ||
| When I am in pain, I keep thinking about how much it hurts. | |||
| 11. Cuando tengo dolor, no dejo de pensar en lo mucho que quiero que pare el dolor. | .587* | ||
| When I am in pain, I keep thinking about how much I want the pain to stop. | |||
| 12. Cuando tengo dolor, no puedo hacer nada por parar el dolor. | .423* | ||
| When I am in pain, there is nothing I can do to stop the pain. | |||
| 13. Cuando tengo dolor, me pregunto si algo grave puede ocurrir. | .757* | ||
| When I am in pain, I wonder whether something serious may happen. |
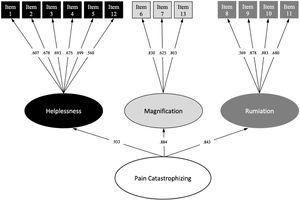
The CFA corroborated that all items loaded on the assumed theoretical factor, showing an optimal factor loading (≥0.560). Thus, we decided to fit a 3-factor CFA model with the 13 items mentioned above. The final model fit the data well: χ245=66.48 (P=.325); CFI=0.993, TLI=0.992; RMSEA=0.022, 95% CI 0.001–0.055; SRMR=0.043. Fig. 2 shows the standardized factor loadings of the final 3-factor solution.
Spanish version of the PCS-C: reliability and floor and ceiling effectsThe final Spanish version of the PCS-C consisted of a total of 13 items. All items were worded as direct, affirmative statements and rated on a 5-point Likert scale (0–4). Therefore, the total score can range from 0 to 52 points. The internal consistency of the scale was excellent (Cronbach α=0.904; 95% CI, 0.880−0.925), with its 3 subscales showing an internal consistency of 0.800 or greater (helplessness, 0.810 [95% CI, 0.759−0.854]; magnification, 0.800 [95% CI, 0.737−0.850]; and rumination, 0.843 [95% CI, 0.797−0.880]). Only 8.7% of the children obtained the lowest possible score on the scale, and no child obtained the highest possible score. Thus, there was no evidence of floor or ceiling effects for the final Spanish version of the PCS-C.
Convergent validityTable 3 presents the data on the correlation of the Spanish version of the PCS-C with all the other measures. Pain interference in the child’s life was the variable most strongly associated with the total score of the PCS-C, with a significant, positive and moderate correlation between the two (r=0.400). Thus, children with high levels of pain interference were children with higher levels of pain catastrophizing. In addition, the PCS-C exhibited a weak correlation with the other pain-related measures in child reports (FPS-R for pain intensity, r=0.217) and parental reports (PPPM, r=0.254; and NRS for Functional Disability, r=−0.186). However, quality of life was only significantly related to the PCS-C in the child version of the PedsQL, with a weak-to-moderate correlation (r=0.303). In short, children with greater catastrophizing perceived more intense pain and a greater deterioration in their quality of life, whereas their parents observed greater changes in their behaviour in association with pain. We did not find a correlation with the number of surgeries or of previous medical consultations.
Convergent validity of the Spanish version of the Pain Catastrophizing Scale for Children (PCS-C).
| Spanish version PCS-C | ||||
|---|---|---|---|---|
| Total Score | Helplessness | Magnification | Rumination | |
| Medical Consultations in the last year | 0.050 | 0.035 | 0.046 | 0.060 |
| Previous Surgeries | 0.014 | −0.002 | 0.002 | 0.019 |
| FPS-R | 0.217** | 0.162* | 0.208** | 0.245** |
| PPPM | 0.254** | 0.256** | 0.186* | 0.236** |
| PROMIS Paediatric Pain Interference | 0.400** | 0.341** | 0.309** | 0.404** |
| Functional Disability | ||||
| Child Response | −0.147 | −0.148 | 0.207* | −0.078 |
| Parent/Guardian Response | −0.186* | −0.153 | −0.182* | −0.197* |
| PedsQL | ||||
| Child form | 0.303** | 0.306** | 0.255** | 0.246** |
| Parent/guardian form | 0.116 | 0.132 | 0.100 | 0.062 |
FPS-R, Faces Pain Scale-Revised; NRS, Numerical Rating Scale; PCS-C, Pain Catastrophizing Scale for Children; PedsQL, Pediatric Quality of Life Inventory; PPPM, Parents Postoperative Pain Measure.
Given the importance of pain catastrophizing and its association with the fear-avoidance model of pain,3 we proposed translating and validating the PCS-C for children and adolescents to Spanish.
Prior to this translation and validation into Spanish, other adaptations of the original PCS-C scale have been made to different languages. Since the final version of the Spanish PCS-C consists of 13 items grouped into 3 factors/subscales, it maintains the original structure, and all items remain in their original factor categories (rumination, magnification and helplessness).9 However, previous validation studies of the PCS-C had found that the 3-factor structure and the total number of the items of the scale were inconsistent.10–12 This inconsistency can be explained by the characteristics of social-cognitive development of children and adolescents, in whom pain catastrophizing may be a developmentally normal, rather than pathological, mental process.29 Reinforcing our results, the studies of the Catalan, French and English versions of the PCS-C support the original 3-factor structure of the scale.13–15 However, the agreement with these studies13,14 was partial, as they found items 8 and 12 problematic due to low factor loading (<0.40). In fact, the results for the Catalan version13 suggested the exclusion of item 8. In contrast to our findings, the results for the Swedish, German and English versions of the PSC-C suggested a 2-factor structure, in addition to the exclusion of some items that appeared problematic.10–12 Specifically, the elimination of items 7 and 8 due to floor/ceiling effects in the English version12 and the elimination of item 8 due to a factor loading of less than 0.4 in the Swedish version.10 Based on these observations, most previous validation studies of the PCS-C appear to agree on the elimination of item 8 (“When I am in pain, I want the pain to go away”) to obtain a better overall model fit.10,12–14 However, our model presented a good fit including item 8, which exhibited an adequate factor loading, and therefore we recommend its inclusion in the Spanish version of the PCS-C. This discrepancy with respect to item 8 could be explained by the fact that our sample was composed of children that were having surgery. Hence, it would be reasonable for these children to expect that pain would be eliminated completely, or greatly reduced, after surgery. This could increase their coping and emotional control skills, which are lesser in children compared to adults.29 In contrast, children included in previous validation studies10,12–14 may have assumed that they must learn to live with pain and appreciated having self-management/coping strategies, but not expected to eliminate the pain completely. This perspective could increase the degree of helplessness perceived by the child and thus contribute to the observed differences in pain catastrophizing processes between the children in our study and those included in previous validations of the PCS-C. This argument is reinforced by the problem previously observed with item 12 (“There is nothing I can do to stop the pain”), which could again be explained by the hopelessness of the children regarding the elimination of the pain. However, this is merely a hypothesis, and further research is required to investigate this issue.
The final Spanish version of the PCS-C exhibited adequate discriminative ability, since no floor or ceiling effects were detected. In addition, the internal consistency of this version in Spanish of the PCS-C was excellent for both the entire scale and its 3 factors. Our results are consistent with those reported for the original version of the scale9 and with those reported in previous validations of the scale in other languages.10,13–15 The small differences observed in the Cronbach α between different versions of the scale might be due to cultural differences regarding how health and wellbeing are conceived and the prognosis of the disease. In our sample, there were children with chronic diseases and with less favourable prognoses, which could have increased the homogeneity of the scores. In addition, the most similar values were found with the Catalan version13 (Cronbach α for Spanish version=0.90; Cronbach α for Catalan version=0.89). Thus, this aspect reinforces our findings, given that Catalonian children share language and culture with Spanish children.
In agreement with previous validation studies,9,11–15 pain catastrophizing as measured by the Spanish version of the PCS-C was associated with pain intensity, disability and pain interference. Specifically, we found a weak correlation was found between child-reported pain catastrophizing and child- and parent-reported pain intensity, which could be due to an increase in anticipatory pain behaviours in our population and to the fact that the symptoms in children had not lasted long enough to change their beliefs or behaviour toward pain.7 The behaviours and attitudes of parents can also influence how children experience pain and facilitate pain disability.8,12 Thus, parental negative behaviours toward pain, as well as an overprotective behaviour toward the child,8 might explain the greater impact of catastrophizing on the child’s functioning and quality of life compared to the perceived pain intensity. Reinforcing this hypothesis, our results show that pain catastrophizing interferes moderately with the child's activities of daily living (pain interference) and quality of life. Furthermore, these correlations support the paediatric fear-avoidance model and might explain how the attitudes and behaviour toward pain of children and parents (such as catastrophizing) increase the impact of pain on their lives and reduce the child’s quality of life.6,7
Finally, pain catastrophizing was not correlated to the number of previous surgeries or medical consultations in the previous year. A possible explanation would be that the reason for the surgical intervention and/or the cause of the child’s pain was not sufficiently intense, disabling or prolonged to promote changes in pain-related beliefs and behaviors.30 Again, more research is needed to better understand this potential relationship.
Limitations and future researchThe study had some limitations. It was conducted on a convenience sample, which could affect the extrapolation of the results to a different group of children. However, the study was performed at a tertiary referral hospital in Spain, which is a paediatric care reference centre and therefore serves patients from every region in Spain. The study was conducted in Spain and its findings showed that the scale had very good psychometric properties, but it is unknown whether these favourable properties would hold if the scale were applied in other Spanish-speaking countries. Finally, the sample analysed in this study consisted exclusively of patients with scheduled surgeries. Due to the complexity of the evaluation and the development of the study, we were unable to evaluate the performance of the scale in other populations, such as children with cancer-related pain or undergoing urgent surgeries. This limitation could restrict the generalization of the results, which suggest that future studies should pursue this line of research.
ConclusionsThe Spanish Version of the Pain Catastrophizing Scale-Children is a valid, reliable scale to assess pain catastrophizing in children and adolescents. Therefore, we recommend its use in research and clinical practice. Further study is warranted to explore its correlations with other paediatric pain-related scales.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical considerationsThe study was approved by the Ethics Committee of the Hospital Universitario 12 de Octubre on December 15, 2020 (CEIm 20/618).
Conflict of interestThe authors have no conflicts of interest to declare.
Author contributionsDoctoral student Ceniza-Bordallo conceived and designed the study, developed the data collection tools, collected the data, conducted the analyses and reviewed and edited the manuscript.
Doctors Martín-Casas and López-de-Uralde-Villanueva collaborated in the conception and design of the study, overseeing and supervising the data collection process. They provided critical insights and intellectual contributions to the manuscript, reviewing and revising it for significant content enhancement.
Doctor Gómez Fraile, MD conducted a thorough evaluation of the manuscript, providing essential intellectual contributions and offering valuable feedback. He also supervised the data collection process and played a critical role in reviewing and revising the manuscript for significant intellectual content enhancement.
We express our deep appreciation for the involvement and interest of all the patients and families that participated in the study. Also, our special thanks to all the nursing staff of the department of surgery of the Hospital Universitario 12 de Octubre as well as the supervisor and staff of the day hospital for their involvement and enormous collaborative effort.