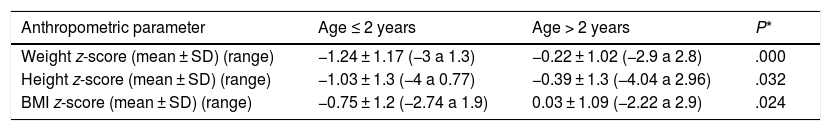European guidelines for the diagnosis of celiac disease (CD) have been updated in 2020. The primary objective was to review the compliance with the diagnostic criteria for CD, according to ESPGHAN 2012. Secondarily, to describe the clinical characteristics of the patients and to assess the changes that would be implied by the application of the new 2020 criteria.
Patients and methodsRetrospective multicenter study in which 10 centers participated. Patients from 0 to 16 years old with a new diagnosis of CD in 2018–2019 were included. Clinical, serological variables and the performance of intestinal biopsy (IB) were collected.
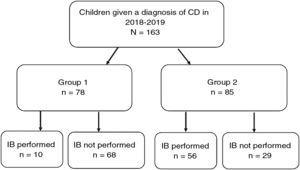
Results163 patients were included (57% female) with a median age of 7.6 years (SD 4.4). The form of presentation was: 47.8% classical, 30.7% no classical and 21.5% asymptomatic, with differences depending on age. Total IgA and anti-transglutaminase IgA antibodies were performed in all centers as the first diagnostic step. IgA anti-endomysial antibodies (EMA) were performed in 80%, and HLA haplotype in 95%. Of the total, 78 cases (47.9%) met criteria for not performing intestinal biopsy (IB). IB was indicated in the remaining 85 patients, but was not performed in 29 cases (17.8%). The performance of IB was lower in the secondary hospitals than in the tertiary ones (p < 0.05).
If we applied the ESPGHAN 2020 criteria, we would disregard the HLA study, and 21 more patients would not have required IB (going from 47.9% to 60.7% of the total).
ConclusionsDiscrepancies are observed in the application of the ESPGHAN 2012 diagnostic criteria due to the different accessibility to EMA and endoscopic IB in secondary centers. With the ESPGHAN-2020 criteria, around 60% of patients will be able to be diagnosed without IB, provided that the determination of EMA is ensured.
Las directrices europeas para el diagnóstico de la enfermedad celiaca (EC) han sido actualizadas en 2020. El objetivo primario fue revisar el grado de cumplimiento de los criterios diagnósticos de EC publicados por la ESPGHAN en 2012. Secundariamente, describir las características clínicas de los pacientes incluidos y valorar los cambios que implicaría la aplicación de los nuevos criterios 2020.
Pacientes y métodosEstudio multicéntrico retrospectivo con participación de 10 centros. Se incluyeron pacientes de 0 a 16 años de nuevo diagnóstico de EC en 2018–2019. Se recogieron variables clínicas, serológicas y realización de biopsia intestinal (BI).
ResultadosIncluimos 163 pacientes (57% mujeres), de edad media 7,6 años (DE 4,4). La forma de presentación fue: 47,8% clásica, 30,7% no clásica y 21,5% asintomáticos, con diferencias según la edad. Todos los centros determinaron IgA total y anticuerpos antitransglutaminasa IgA como primer escalón diagnóstico. Los anticuerpos antiendomisio IgA (AAE) se realizaron en el 80% y el haplotipo HLA en el 95%. Del total, 78 casos (47,9%) cumplieron criterios para no realizar BI. En los restantes 85 pacientes estaba indicada BI, no realizándose en 29 casos (17,8%). La realización de BI fue menor en los hospitales secundarios que en los terciarios (p < 0,05).
Si aplicáramos los criterios ESPGHAN-2020, prescindiríamos del estudio del HLA y 21 pacientes más no hubieran precisado BI (pasando de un 47,9% a un 60,7% del total).
ConclusionesSe observan discrepancias en la aplicación de los criterios diagnósticos ESPGHAN-2012 debido a la diferente accesibilidad a los AAE y BI endoscópica en centros secundarios. Con los criterios ESPGHAN-2020, en torno a un 60% de los pacientes podrán ser diagnosticados sin precisar BI, siempre que se asegure la determinación de AAE.
Coeliac disease (CD) is an immune-mediated disorder elicited by gluten intake and related prolamins in genetically susceptible individuals. It is characterised by the presence of a variable combination of clinical manifestations, specific antibodies, HLA DQ2 or DQ8 haplotypes and enteropathy.1
When it comes to the clinical presentation of CD, a growing number of cases with mild or nonspecific symptoms or without symptoms are being detected.2 The age at diagnosis has also changed, so that CD has gone from being a disease with a childhood onset to a disease diagnosed in every age group.3
Advances in our understanding of the disease, especially as regards specific antibodies, have also brought changes to the criteria used to diagnose CD over the years.4 These have resulted from the identification of tissue transglutaminase (tTG) antibodies and endomysial antibodies (EMA), the evidence on the strong correlation between high titres of tTG antibodies and mucosal severity5,6 as well as the identification of EMA as a marker of disease.7 The accuracy of the serological markers that are currently available has led to a re-evaluation of the role of the intestinal biopsy (IB) in the diagnosis of CD.8,9
Thus, in 2012 the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) published guidelines for diagnosis of CD, establishing that the IB could be omitted in select cases: symptomatic patients with high anti-tTG titres with levels greater than 10 times the upper limit of normal (ULN), a positive result for EMA in a second blood sample and the HLA DQ2 or DQ8 haplotype.1 These recommendations were updated in 2020, so that a no-biopsy diagnosis can also be made in asymptomatic patients, with possible omission of HLA testing, in cases meeting the serological criteria (elevation of tTG antibodies > 10 times the ULN and positive EMA result in a different blood sample).10
Our primary objective was to assess the degree of adherence to the 2012 ESPGHAN criteria in a cohort of paediatric patients given a CD diagnosis in 2018 or 2019 in a multicentre study. The secondary objectives were to describe the clinical and epidemiological characteristics of the sample and assess the changes in diagnosis that would result from the application of the recently updated criteria published in 2020.
Patients and methodsWe conducted a multicentre retrospective study with participation of the paediatric gastroenterology units of 10 hospitals (2 tertiary care and 8 secondary care hospitals). We included patients aged 0–16 years given the initial diagnosis of CD in the 2018–2019 period. We collected data on epidemiological, clinical, serological and genetic characteristics. We also documented whether patients underwent an endoscopic IB, grading histological features based on the Marsh classification modified by Oberhuber.11 We excluded patients who had started a gluten-free diet before the diagnosis was completed.
We classified the clinical presentation as “asymptomatic”, “typical” (signs and symptoms of malabsorption: diarrhoea, weight loss and/or abdominal distension) or “non-classical” (symptomatic but without signs or symptoms of malabsorption).12
Anthropometric data were collected at the time of diagnosis. We calculated the weight, height and body mass index (BMI) z-scores using the World Health Organization (WHO)growth standards.13,14
To evaluate the adherence to the 2012 ESPGHAN criteria, we divided patients in 2 groups. Group 1 included those that met the criteria to omit the IB: compatible symptoms, anti-tTG at or above 10 times the ULN, separate blood sample positive for EMA and HLA DQ2 or DQ8 haplotype. Group 2 included the rest of cases, in which an IB would be required for diagnosis due to not meeting at least 1 of these criteria.1
To assess the changes that would derive from applying the 2020 ESPGHAN criteria for diagnosis, we excluded the symptoms and HLA haplotype as requirements for membership in group 1 while maintaining the other criteria.10
We conducted the statistical analysis with the Statistical Package for the Social Sciences (SPSS), version 22.0. Statistical significance was defined as a 5% probability of type 1 error (α = 0.05). We performed a descriptive analysis of the data. We summarised nominal and ordinal data as absolute frequencies and percentages. We summarised continuous variables with measures of central tendency (mean and median) and of dispersion (standard deviation [SD]). Nominal variables were compared with the chi square test or the Fisher exact test. Quantitative variables were compared with the Student t test in case of a normal distribution or otherwise with the Mann-Whitney U and Kruskal–Wallis nonparametric tests.
The study adhered to the ethical principles of the most recent Declaration of Helsinki (www.wma.net), the Good Clinical Practice guidelines of the International Council for Harmonisation (GPC/ICH) and current Spanish law (www.aemps.es/).
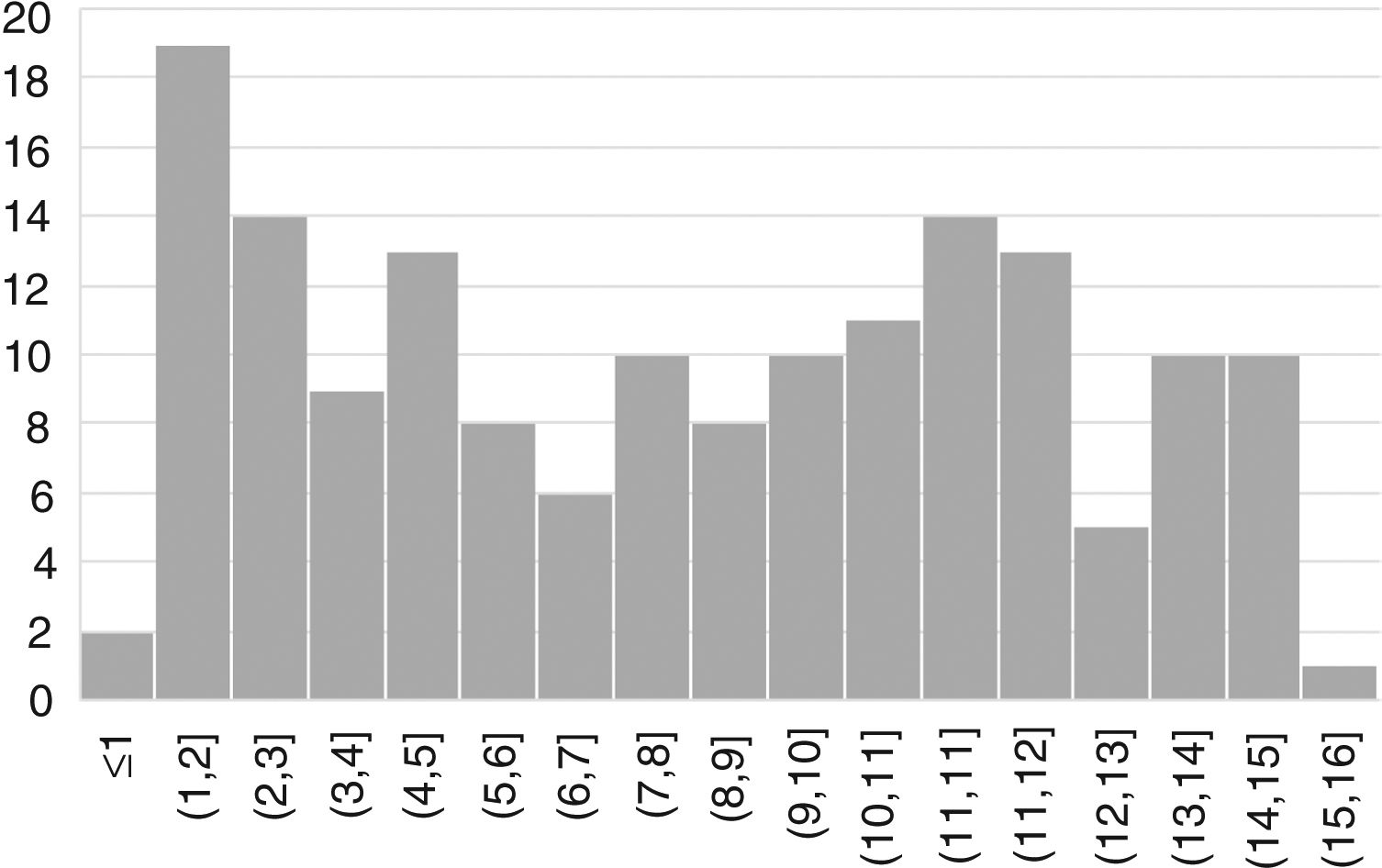
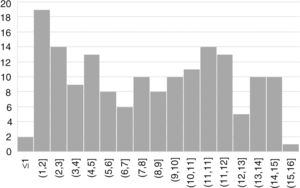
ResultsWe included 163 patients (57% female) with a mean age of 7.6 years (SD, 4.4) (Fig. 1).
In 110 cases (67.5%), patients were evaluated due to manifestations suggestive of CD, in 16 (9.8%) due to screening of risk groups and in 37 (22.7%) for other reasons. Twenty percent of the children given a diagnosis of CD in this period had a family history of CD (12% in first-degree relatives and 8% in second-degree relatives). The comorbidities found in the sample were diabetes mellitus in 5 children, autism spectrum disorder in 2 and Down syndrome in 2. We did not find any other disorders associated with CD.
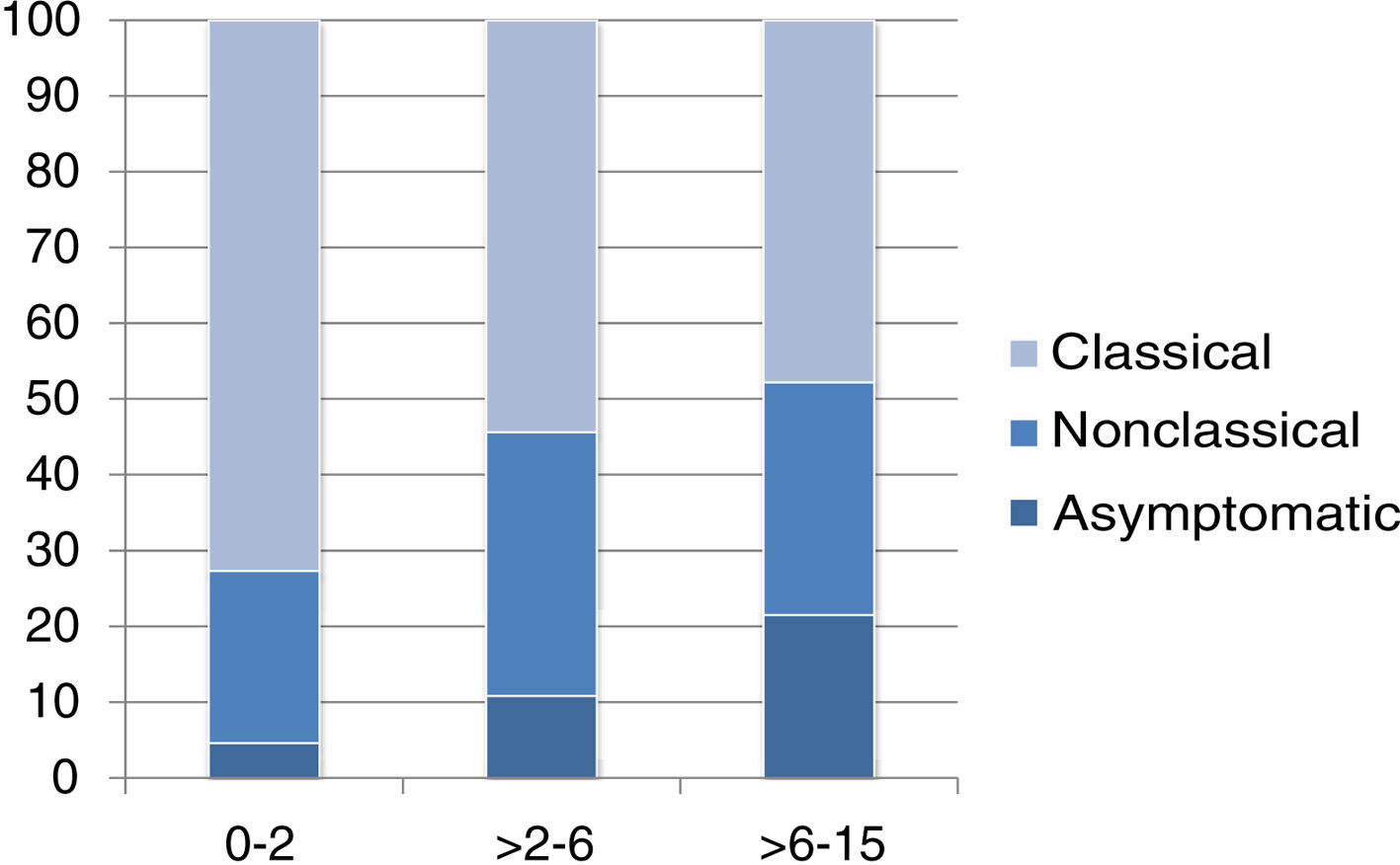
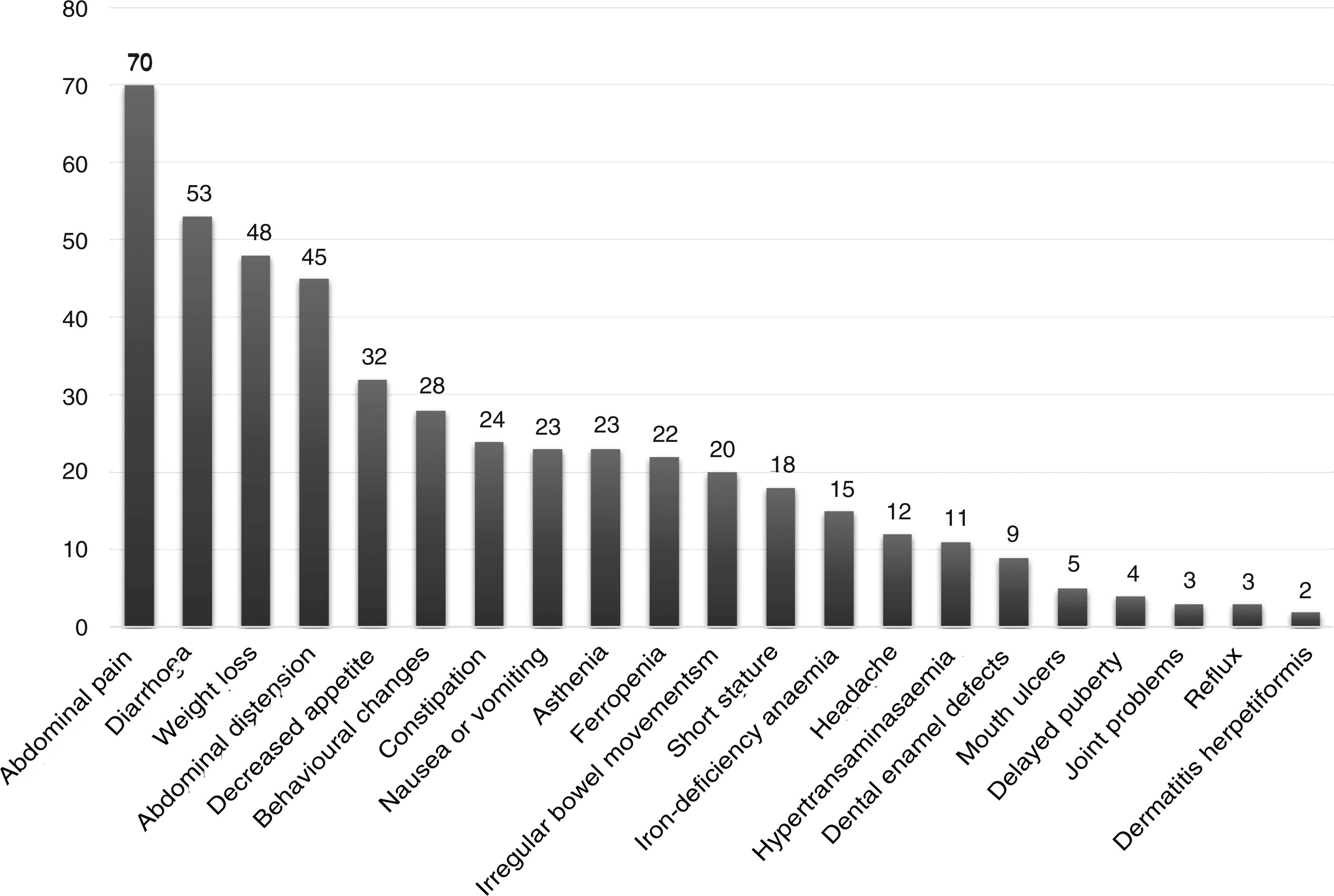
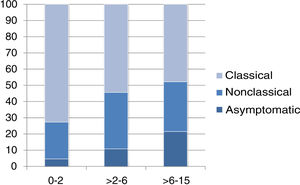
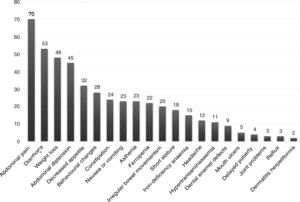
The presentation was classical in 78 patients (47.8%), non-classical in 50 (30.7%) and asymptomatic in 35 (21.5%). We found significant differences in the form of CD based on age, as classical presentations were more frequent in children aged less than 2 years and asymptomatic presentations in children aged more than 6 years (P = .006) (Fig. 2). Fig. 3 summarises the presenting symptoms at diagnosis, the most frequent of which was chronic abdominal pain.
The analysis of the anthropometric variables at the time of diagnosis revealed lower z-scores in patients aged 2 years or less, a difference that was statistically significant (Table 1).
Comparison of z-scores for anthropometric measurements at the time of diagnosis in children aged 2 years or less and older children.
| Anthropometric parameter | Age ≤ 2 years | Age > 2 years | P* |
|---|---|---|---|
| Weight z-score (mean ± SD) (range) | −1.24 ± 1.17 (−3 a 1.3) | −0.22 ± 1.02 (−2.9 a 2.8) | .000 |
| Height z-score (mean ± SD) (range) | −1.03 ± 1.3 (−4 a 0.77) | −0.39 ± 1.3 (−4.04 a 2.96) | .032 |
| BMI z-score (mean ± SD) (range) | −0.75 ± 1.2 (−2.74 a 1.9) | 0.03 ± 1.09 (−2.22 a 2.9) | .024 |
In all hospitals, the first step of diagnosis was measurement of the total IgA and anti-tTG IgA levels, a test that was positive in every case except in 4 patients with IgA deficiency, with levels more than 10 times the ULN in 134 patients (82%). The EMA test was performed in 130 patients (79.6% of the total) and was positive in 95.5%. Of all the EMA tests, 25 (19%) were not performed in a different blood sample than the one used to measure tTG IgA. The HLA haplotype was documented in 154 patients (95%), of who 83% had the DQ2, 9% the DQ8, 6% the DQ2/DQ8 and 2% the DQ2A or DQ2B allele.
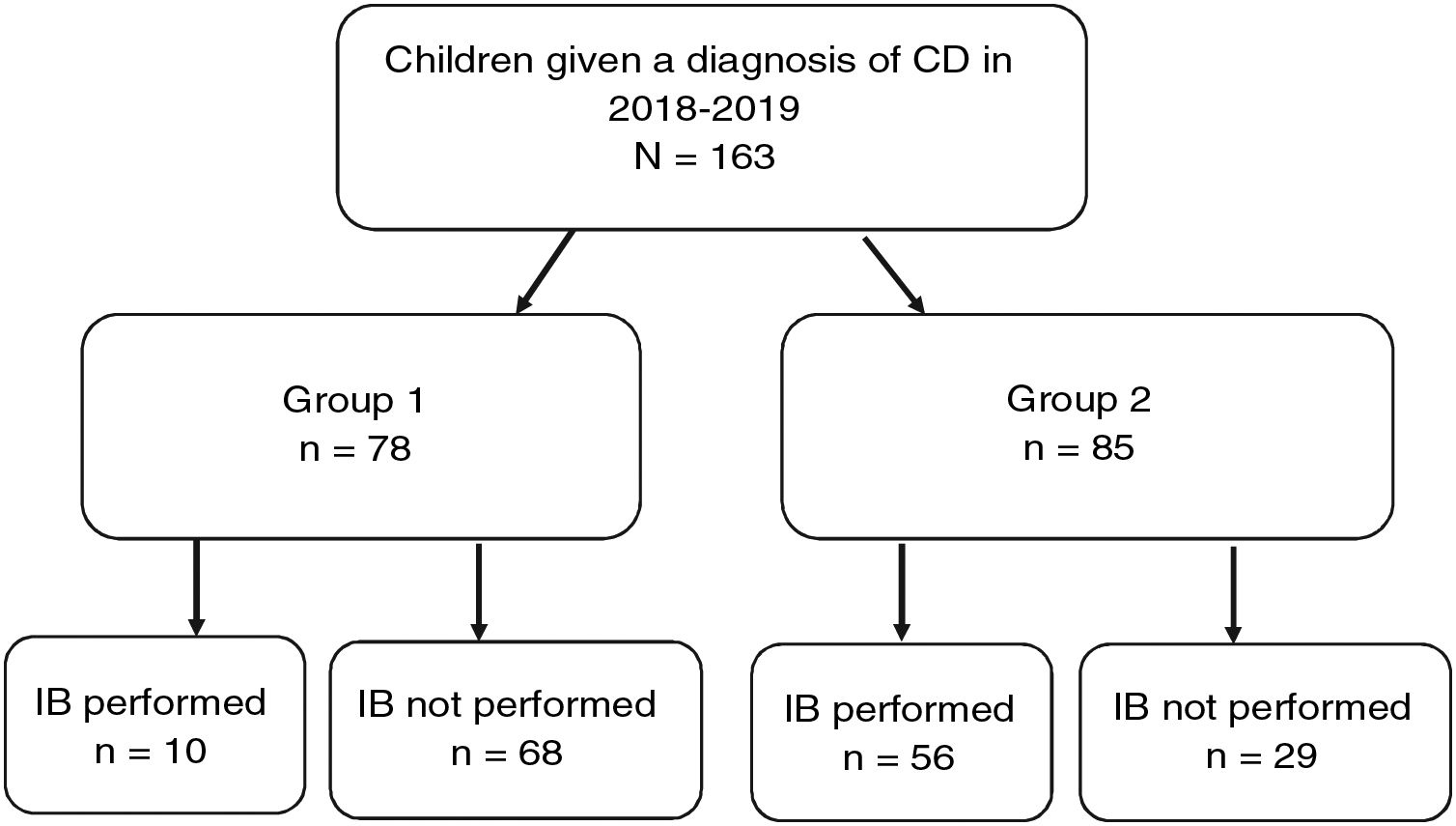
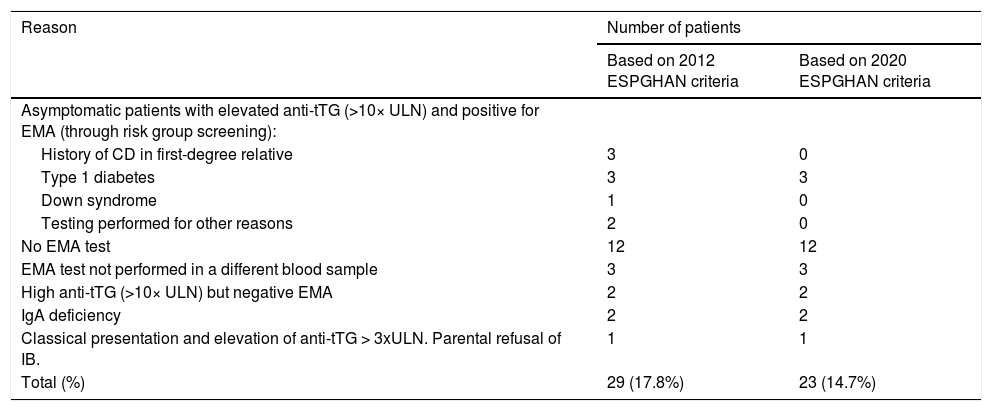
Fig. 4 provides a graphic representation of the use of IB based on the ESPGHAN 2012 criteria. Group 1, which included patients that met the criteria for a no-biopsy diagnosis, comprised 78 cases (47.9% of the total). In this group, an IB was performed in 10 patients due to the mildness of the symptoms (in many cases, the sole symptom was nonspecific abdominal pain). Group 2 included the remaining 85 patients in who IB was indicated because they did not meet all the required criteria. However, in 29 cases (17.8% of the total) IB was not performed despite being indicated. Table 2 presents details on these 29 patients. We found that IB was performed significantly less frequently in secondary versus tertiary care hospitals, with a proportion of IB of 29.1% in the former and 58.3 in the latter (P < .05). The histological features in patients that underwent an IB based on the Marsh classification modified by Oberhuber were graded as type 2 in 2 patients (3%), type 3a in 15 (22.7%), type 3b in 28 (42.4%) and type 3c in 21 (31.8%). We did not find a correlation between the anti-tTG titres and histological classification.
Performance or lack of performance of intestinal biopsy (IB) and adherence to the ESPGHAN 2012 diagnostic criteria.
Group 1 Patients that met the ESPGHAN 2012 criteria for diagnosis without an IB (symptoms, anti-tTG ≥ 10× ULN, positive EMA test in a different blood sample, compatible HLA allele).
Group 2: Patients that did not meet the ESPGHAN 2012 criteria for diagnosis without an IB.
Cases in which intestinal biopsy was indicated for diagnosis of coeliac disease but it was not performed.
| Reason | Number of patients | |
|---|---|---|
| Based on 2012 ESPGHAN criteria | Based on 2020 ESPGHAN criteria | |
| Asymptomatic patients with elevated anti-tTG (>10× ULN) and positive for EMA (through risk group screening): | ||
| History of CD in first-degree relative | 3 | 0 |
| Type 1 diabetes | 3 | 3 |
| Down syndrome | 1 | 0 |
| Testing performed for other reasons | 2 | 0 |
| No EMA test | 12 | 12 |
| EMA test not performed in a different blood sample | 3 | 3 |
| High anti-tTG (>10× ULN) but negative EMA | 2 | 2 |
| IgA deficiency | 2 | 2 |
| Classical presentation and elevation of anti-tTG > 3xULN. Parental refusal of IB. | 1 | 1 |
| Total (%) | 29 (17.8%) | 23 (14.7%) |
Anti-tTG, tissue transglutaminase antibodies; EMA, endomysial antibodies; IB, intestinal biopsy; IgA, immune globulin A; ULN, upper limit of normal.
When we applied the ESPGHAN 2020 criteria, we found that HLA typing could have been omitted in 138 patients: the 154 in who it was performed, excluding the 16 in which it was indicated due to screening of risk groups. On the other hand, another 21 patients in the sample would not have required an IB because despite being asymptomatic, they met all the serological criteria, which would correspond to an increase from 78 (47.9%) to 99 patients (60.7%). In addition, of the 29 cases incorrectly diagnosed based on the 2012 guidelines, 9 corresponded to asymptomatic patients with very high anti-tTG titres and positive EMA results in a different blood sample. Excluding 3 that had diabetes, the diagnosis would have been appropriate in the remaining 6 based on the 2020 guidelines, as an IB would not be required with the current criteria. Thus, the number of diagnoses that did not adhere strictly to the guidelines would decrease from 17.8% to 14.7%.
DiscussionThe primary objective of our study was to evaluate the degree of adherence to the ESPGHAN criteria for diagnosis of CD and the secondary objectives were to analyse the current clinical and epidemiological characteristics of CD in paediatric patients in our area in a large sample obtained from different hospitals. By including tertiary and secondary care hospitals we sought to reflect the reality of how CD is being diagnosed based on the changes brought on by the publication of the ESPGHAN 2012 criteria. We found that CD is diagnosed at all ages, and that the mean age of our patients was greater compared to the mean reported by other authors,15,16 which reflects the increasing trend of diagnoses at later ages.17–19 We also found a predominance of female patients, in a percentage that was similar to those described in other studies.15,19–22 The high percentage of patients with a family history of CD (20%) suggests that hospitals in our region actively investigate the history of families of diagnosed cases, and this proportion was consistent with the previous literature.23,24 In addition, diabetes25 and Down syndrome26 are the most frequently reported comorbidities. We did not find any of the other comorbidities previously described, such as Turner syndrome or Williams syndrome, probably due to the lower prevalence of these disorders.
When it came to the clinical presentation, we found a predominance of the classical form (47.8%), manifesting with chronic abdominal pain, chronic diarrhoea, weight loss and abdominal distention, which was also the most frequent presentation in children aged less than 2 years, as described in the previous literature.20,24,27,28 We ought to highlight the substantial proportion of patients that presented with less typical signs or symptoms (30.7%), such as ferropenia, dental enamel defects, aphthae, hypertransaminasaemia, short stature, among others. We found 21.5% of asymptomatic cases, in which serological testing was performed due to a positive family history, membership in a risk group or other reasons, with a higher proportion of such cases in older patients. Asymptomatic presentations are increasing in frequency, ranging from 16% to 28% of total cases depending on the study.17,29
We ought to highlight that the higher frequency of malabsorption syndrome in young children plays a role in the greater impact on growth parameters also observed in this age group, with significantly lower z-scores for weight, height and BMI compared to children aged more than 2 years, as has been previously described.16,20
Now focusing on the diagnosis process, we found that all hospitals had access to the anti-tTG IgA test, used for the initial screening in every case. We also found a high percentage of patients (82%) with anti-tTG IgA titres above 10 times the ULN, which was higher compared to other studies.30 However, when it came to the EMA test, which is an indirect immunofluorescence assay that requires trained personnel and is more costly and labour-intensive than the anti-tTG test,31 we found that some hospitals had limited access to it. We also found that the recommendation to perform the EMA test in a different blood sample than the one used for the anti-tTG test was not followed in 19% of cases.
As for HLA typing, the technique was widely available in the participating hospitals and typing was performed in 95% of patients. The HLA allele distribution in our series was consistent with the previous literature save for a slightly higher frequency of the DQ8 allele (9% compared to the 5% previously reported for the Spanish population).20,32 In agreement with the 2020 diagnostic criteria, we found that HLA typing did not increase the accuracy of the diagnosis if all other criteria were met.10 Therefore, since this is an expensive technique and it is mainly useful on account of its negative predictive value, it has been proposed that it be reserved for screening of risk groups or in case of diagnostic uncertainty.
The percentage of cases that met the criteria for diagnosis without an IB based on the 2012 criteria was 47.9%, which was consistent with other studies that found that the IB could be avoided in approximately half the patients.24 However, in 10 of these cases the patient underwent an IB on account of mild or nonspecific symptoms, with these cases clustering in tertiary care hospitals, probably due to the greater access to endoscopy in these centers. Performance of IB despite the presence of symptoms may have been justified in patients with a non-classical presentation, which may involve less specific symptoms compared to the typical presentation.24 In our study, most of the 29 cases in which IB was not performed despite being indicated corresponded to cases diagnosed in regional hospitals, patients with very high anti-tTG titres but asymptomatic, centers in which EMA testing was not available or cases in which the EMA test was not performed in a different blood sample.
Applying the 2020 criteria, we found that the diagnosis would be correct in 6 of the patients in this group, as they were asymptomatic and met the serological criteria. In the rest of cases, the diagnosis should be reconsidered and based on the course of disease, the possibility of conducting a gluten challenge during the follow-up should be contemplated. This underscores the importance of having the EMA test available in every hospital, as diagnosis of CD without performance of an IB requires strict application of the established criteria to avoid overdiagnosis, as the latter entails unnecessary implementation of a gluten-free diet for life.
The main limitation of this study is that due to its retrospective and multicentre design, the anti-tTG and EMA tests were not all performed in the same laboratory.
In conclusion, CD in our region is diagnosed in a broad age range. The classical form continues to be the most frequent presentation, especially in children aged less than 2 years, and non-classical and asymptomatic forms are more frequent at older ages. When it comes to its diagnosis, the discrepancies we found relative to the ESPGHAN 2012 criteria were mainly due to differences between hospitals in the access to EMA tests and endoscopic IB. With the current ESPGHAN 2020 criteria, the proportion of cases that could have been diagnosed without an IB would be of approximately 60%, so that the proportion of patients whose diagnosis was not in adherence would have decreased from 18.4% to 14.7%. Nevertheless, caution is of the essence, and serological criteria must be applied strictly, ensuring measurement of EMA levels in every hospital. Larger and prospective studies are required to assess and validate these new diagnostic criteria.
FundingThe study did not receive any form of funding.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Crehuá-Gaudiza E, Barrés Fernández A, Jovaní Casano C, Latorre Tejerina M, Largo Blanco EM, Moreno Ruiz MA, et al. Diagnóstico de enfermedad celiaca en la práctica clínica: presente y futuro. An Pediatr (Barc). 2021;94:223–229.