Malaria is considered to be the fourth leading cause of infant mortality after pneumonia, complications related to premature birth, and perinatal asphyxia.
Material and methodsA retrospective and descriptive study of cases of malaria confirmed and treated by the Paediatric Infectious Diseases Unit (age lower than 15 years) at the La Fe Hospital, Valencia, over the period 1993–2015.
ResultsA total of 54 cases of paediatric malaria were diagnosed in the period 1993–2015, with 51.8% of these occurring in males, and 46.2% of patients were aged below 5 years. The majority of children came from Equatorial Guinea (68.5%). Only 5.6% had received antimalarial prophylaxis. Plasmodium falciparum was found to be the causal species in 81.4% of cases. Seven patients (13%) presented with complicated malaria. The most widely used treatment was quinine, either alone or in combination with other drugs. Atovaquone/proguanil was used from 2010 onwards and was indicated in 20.3% of the patients. The combination of artesunate/piperaquine/dihydroartemisinin began to be used in 2013. No deaths or relevant side effects were reported, and the clinical response was favourable in all children (100%).
ConclusionsMalaria is still a prevalent disease in our population, a consequence of immigration, and tourism to endemic countries. Malaria should be considered as a likely diagnosis in a febrile child who comes from, or has travelled to, an endemic region in the past year.
La malaria es considerada la cuarta causa de mortalidad infantil después de la neumonía, las complicaciones por parto prematuro y la asfixia perinatal.
Material y métodosEstudio retrospectivo y descriptivo de los casos de paludismo confirmados y tratados en la Unidad de Enfermedades Infecciosas Pediátricas (edad inferior a 15 años) del Hospital La Fe (Valencia) en el período comprendido entre 1993 y 2015.
ResultadosDurante el período 1993-2015 se diagnosticaron 54 casos de malaria infantil, el 51,8% en varones. El 46,2% eran menores de 5 años. La mayoría de los niños procedían de Guinea Ecuatorial (68,5%). Solo en el 5,6% de los pacientes se pudo constatar que recibieran profilaxis antimalárica. Se evidenció que Plasmodium falciparum fue la especie causal del 81,4% de los episodios. Siete casos (13%) presentaron malaria complicada. El tratamiento más empleado fue la quinina, sola o en combinación con otros fármacos: atovacuona-proguanil fue empleada a partir del año 2010 y estuvo indicada en el 20,3% de los pacientes. A partir del año 2013 se inició la utilización de: artesunato, piperaquina y dihidroartemisina. No hubo mortalidad ni efectos adversos relevantes, siendo la respuesta clínica favorable en el 100% de los niños.
ConclusionesLa malaria sigue siendo una enfermedad vigente en nuestra población, consecuencia de la inmigración y del turismo a países endémicos. Debe ser considerada como diagnóstico probable ante un niño febril que procede o ha viajado a un área endémica en el último año.
Malaria is the parasitic disease with the highest incidence and causing the greatest mortality worldwide. It is caused by protozoa of the genus Plasmodium and transmitted through the bite of female mosquitoes of the genus Anopheles. Approximately 175 Plasmodium species are currently known, of which 5 are frequently involved in human disease: P. falciparum, P. vivax, P. ovale, P. malariae and P. knowlesi. Malaria is endemic in 104 countries in tropical and subtropical regions worldwide, although the number of cases is particularly high in South America, Central America (Dominican Republic, Haiti), Africa and Asia (India, Southeast Asia and Middle East).
In 2015, there were 214 million new cases of malaria diagnosed worldwide and a total of 438 000 related deaths (78% of them in children aged less than 5 years, according to data from the World Health Organization). Of all deaths, 92% occurred in Africa, followed in frequency by Southeast Asia (6%) and the Eastern Mediterranean (2%). However, 80% to 90% of new cases and deaths occur in Sub-Saharan Africa (mainly Equatorial Guinea) and Southeast Asia (mainly India) due to deficiencies in hygiene, sanitation and medical care in these countries. The same year, the estimated number of deaths caused by malaria in children aged less than 5 years was 303000 (range, 165000–450000). Malaria is considered the fourth leading cause of death in children following pneumonia, complications of preterm birth and perinatal asphyxia.
In 1964, Spain was declared to be free of malaria by the World Health Organization. Since then, between 400 and 600 cases have been notified per year, most of them cases of imported malaria. Increases in immigration and international travel to malaria-endemic countries will bring on an increase in the number of cases in upcoming years.1–5
In the study presented here, we reviewed all cases of malaria in children diagnosed at the Hospital Universitario y Politécnico (HUP) La Fe (the tertiary referral hospital of the Valencian Community) in the past 22 years (1993–2015) with the aim of analysing clinical and epidemiological characteristics and specific aspects of diagnostic testing and the different treatments used against this disease.
MethodsWe conducted a retrospective, observational and descriptive study of cases of malaria in children managed in the Department of Paediatric Infectious Diseases of the HUP La Fe (Valencia). The inclusion criteria were age 1 month to 15 years, management between 1993 and 2015 and diagnosis of malaria based on positive microbiological detection of the parasite and compatible clinical manifestations. We ought to highlight that all included cases corresponded to patients admitted to our department. We excluded patients with a diagnosis of malaria admitted to other departments of the hospital, since malaria was not the disease that predominated in these patients.
The microbiological diagnosis was made through the following methods: detection of Plasmodium antigen by flow chromatographic immunoassay of blood specimens (OnSite Malaria Pf/Pan Ag Rapid Test, Biotech Inc), microscopic examination of thin smears of peripheral blood prepared with the Giemsa stain with calculation of the percentage of parasitaemia, and nucleic acid detection by an in-house PCR test with initial DNA amplification and subsequent analysis of obtained fragments with agarose gel electrophoresis.
For each case, we collected data on demographic variables (age, sex and country of origin), clinical variables (duration of fever to diagnosis, other salient signs and symptoms detected in the physical examination), relevant findings of diagnostic tests, previous prophylactic treatment, identified Plasmodium species and percentage parasitaemia. We also analysed the treatments used, responses to treatment and adverse events. We reviewed the electronic health records of the patients using software of the HUP La Fe, with anonymization of all collected data.
ResultsThe HUP La Fe is the tertiary referral hospital of the Valencian Community. It has a catchment population of 280754 inhabitants, of who 40804 are children, and 146 paediatric beds.
During the 1993–2015 period, there were 54 diagnoses of malaria in children, corresponding to a mean of 2 admissions per year. In the 1993–1996 period, only 2 cases were detected. On the other hand, 2003, 2010 and 2013 were the years with the highest number of admissions (8, 4 and 5 cases, respectively).
Of the total cases, 46.29% (25 cases) corresponded to children aged less than 5 years, while only 18.5% (10 cases) occurred in children aged more than 10 years. The mean age was 6 years, with a standard deviation (SD) of 3.77. Of all these patients, 51.85% (28 cases) were male.
Most patients were from Equatorial Guinea (68.5%) or from Spain (25.9%); a minority of patients were from Ghana, Gambia or Senegal (1.85% of patients each).
Previous episodes of malaria were reported by 46.3% of the patients. We found evidence of antimalarial prophylaxis in 5.6% of patients, all of who had completed the prescribed course of treatment: chloroquine or mefloquine for 2 weeks prior to travelling to an endemic area, throughout the trip and for 4 weeks after returning.
When it came to comorbidities, we found that 9.3% reported a history of typhoid fever and 5.6% sickle cell disease. We also found 1 case with a history of hepatitis B, 1 case with cancer and 1 case of female genital mutilation.
We found no differences in the frequency of subsequent episodes of illness based on the history of prophylaxis or the presence or absence of comorbidities.
From a clinical standpoint, the most frequent symptom was fever (temperature recorded in the emergency department of the hospital), found in 52 patients (96%). The duration of fever varied. When we analysed the time elapsed from onset, we found that patients presented most frequently with fever lasting 1 day (36%), 3 days (19%) or 9 days (9%).
Other clinical manifestations, in order of decreasing frequency, were vomiting (35%), diarrhoea (31%), headache (29%), abdominal pain (24%) and, in lower proportions, respiratory symptoms and chills. The physical examination revealed the following signs: hepatomegaly (59%) and splenomegaly (51%). Other less frequent findings were systolic murmur and pallor.
The most frequent relevant finding in the complete blood count was anaemia (64.8%), defined as a haemoglobin concentration of less than 11g/dL. The mean concentration of haemoglobin in the sample under study was 10g/dL (SD, 1.76). In addition, thrombocytopenia (<150000platelets/mm3) was found in 9 children (17%). Leukopenia (<5000white blood cells/mm3) was infrequent, as it occurred in only 3 of the patients included in the analysis (5.6%).
In the chemistry tests, the most frequent abnormal finding was elevation of acute phase reactants, including C-reactive protein (in 37%), with a mean level of 5mg/dL (SD, 5.17), and the erythrocyte sedimentation rate (in 27.7%), with a mean level of 39mm/h (SD, 28.7). Other recorded abnormalities included elevation of lactate dehydrogenase (in 14.8%), with a mean level of 245IU/L (SD, 381), and indirect bilirubin (in 5.6%), with a mean concentration of 1.4mg/dL (SD, 0.9).
The analysis of the aetiological diagnosis showed that P. falciparum was the causative agent in 81.4% of episodes, followed by P. vivax (7.4%) and P. malariae (1.85%). Five episodes (9.3%) involved more than 1 species of Plasmodium, with detection of P. falciparum and P. vivax in 4 cases and P. falciparum and P. ovale in 1 case.
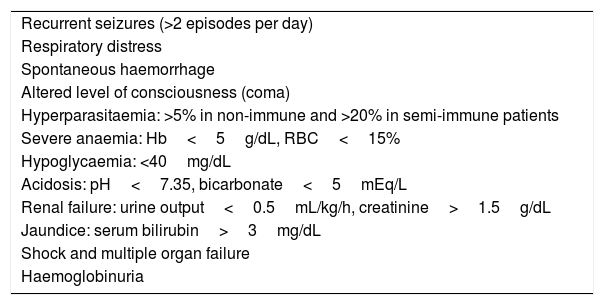
The observed level of parasitaemia varied: 12 patients (22%) had low parasitaemia (<1% of infected red blood cells [RBCs] or <20000trophozoites/L), another 22% had moderate parasitaemia (1–5% or 20000–50000trophozoites/L) and 13% high parasitaemia (>5% or >50000trophozoites/L). In the last group (7 cases), all patients met some of the criteria for complicated malaria (Table 1). Data on this variable were not available for 22 patients (41%). None of the patients required admission to the intensive care unit.
Criteria for severe malaria in children.
| Recurrent seizures (>2 episodes per day) |
| Respiratory distress |
| Spontaneous haemorrhage |
| Altered level of consciousness (coma) |
| Hyperparasitaemia: >5% in non-immune and >20% in semi-immune patients |
| Severe anaemia: Hb<5g/dL, RBC<15% |
| Hypoglycaemia: <40mg/dL |
| Acidosis: pH<7.35, bicarbonate<5mEq/L |
| Renal failure: urine output<0.5mL/kg/h, creatinine>1.5g/dL |
| Jaundice: serum bilirubin>3mg/dL |
| Shock and multiple organ failure |
| Haemoglobinuria |
Adapted from Maitland K, Nadel S, Pollard AJ, et al. Management of severe malaria in children: proposed guidelines for the United Kingdom. BMJ. 2005;331:334–43.
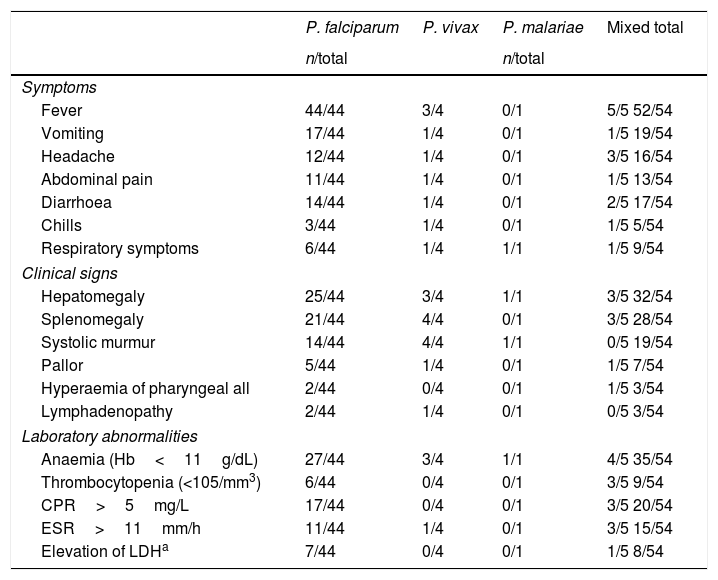
Table 2 summarises the clinical manifestations, findings of the physical examination and results of laboratory tests in our sample by species of Plasmodium.
Clinical presentation and results of diagnostic tests by Plasmodium species.
| P. falciparum | P. vivax | P. malariae | Mixed total | |
|---|---|---|---|---|
| n/total | n/total | |||
| Symptoms | ||||
| Fever | 44/44 | 3/4 | 0/1 | 5/5 52/54 |
| Vomiting | 17/44 | 1/4 | 0/1 | 1/5 19/54 |
| Headache | 12/44 | 1/4 | 0/1 | 3/5 16/54 |
| Abdominal pain | 11/44 | 1/4 | 0/1 | 1/5 13/54 |
| Diarrhoea | 14/44 | 1/4 | 0/1 | 2/5 17/54 |
| Chills | 3/44 | 1/4 | 0/1 | 1/5 5/54 |
| Respiratory symptoms | 6/44 | 1/4 | 1/1 | 1/5 9/54 |
| Clinical signs | ||||
| Hepatomegaly | 25/44 | 3/4 | 1/1 | 3/5 32/54 |
| Splenomegaly | 21/44 | 4/4 | 0/1 | 3/5 28/54 |
| Systolic murmur | 14/44 | 4/4 | 1/1 | 0/5 19/54 |
| Pallor | 5/44 | 1/4 | 0/1 | 1/5 7/54 |
| Hyperaemia of pharyngeal all | 2/44 | 0/4 | 0/1 | 1/5 3/54 |
| Lymphadenopathy | 2/44 | 1/4 | 0/1 | 0/5 3/54 |
| Laboratory abnormalities | ||||
| Anaemia (Hb<11g/dL) | 27/44 | 3/4 | 1/1 | 4/5 35/54 |
| Thrombocytopenia (<105/mm3) | 6/44 | 0/4 | 0/1 | 3/5 9/54 |
| CPR>5mg/L | 17/44 | 0/4 | 0/1 | 3/5 20/54 |
| ESR>11mm/h | 11/44 | 1/4 | 0/1 | 3/5 15/54 |
| Elevation of LDHa | 7/44 | 0/4 | 0/1 | 1/5 8/54 |
Other parasitic infections were also found during hospitalisation, mainly detection of Trichuris trichiura (15% of the total patients), Ascaris lumbricoides (15%), Giardia lamblia (7.5%) or Enterobius vermicularis (1.8%) in stool samples. Other concomitant and isolated findings were impetigo caused by Streptococcus pyogenes, positive hepatitis B surface antigen test in the context of hypertransaminasaemia in 1 patient, a positive tuberculin test in 1 patient and a positive pregnancy test in 1 female patient.
The treatment used most frequently was quinine, alone or in combination with other drugs: a combination with clindamycin was used in 16 cases (29.6%), in 1 case associated to primaquine in the context of concomitant parasitic infection by P. vivax. Seven patients (13%) received combined therapy with pyrimethamine–sulfadoxine, in 1 case combined with primaquine due to mixed infection by P. falciparum and P. vivax. Six children (11.1%) received quinine with chloroquine, combined with primaquine in 3 due to mixed infection (P. vivax). In 5 patients (9.3%), it was combined with doxycycline, with addition to primaquine in 1 (due to technical difficulties in identifying the involved Plasmodium species). Four patients (7.4%) received quinine as monotherapy, with addition of mebendazole in 1 patient due to detection of oxyuriasis in stool. A combination of quinine, sulfadoxine and trimethoprim was used in 3 cases (5.6%).
Atovaquone-proguanil started being used in 2010 and prescribed to 20.3% of patients. In one patient, it was combined with cefotaxime for treatment of a concurrent bacterial infection, in another with quinine; and in a third patient with primaquine on account of mixed infection by P. falciparum and P. ovale. In 2013, the use of artesunate, piperaquine and dihydroartemisinin was introduced in our hospital (2 cases; 3.7%).
The mean duration of treatment was 8 days. Only 2 patients required second-line treatment (3.7%). None of the patients died or developed relevant adverse effects, and the response to treatment was good in 100% of cases.
DiscussionMalaria is one of the most important infectious diseases in terms of morbidity and mortality. We are currently witnessing a nearly exponential growth in the immigrant population, the number of international adoptions and travel to tropical and developing countries. For this reason, we believe that it is relevant to describe the clinical and epidemiological characteristics of this disease, as well as the changes in the diagnostic and therapeutic approach to malaria throughout the study period in the city of Valencia, one of the largest in terms of population in Spain.
This review also describes the characteristics of children admitted to our hospital (the tertiary referral hospital of the Valencian Community) with a diagnosis of malaria in the past 22 years. Nearly half of the patients were aged less than 5 years, an important finding given that malaria is responsible for 8% of all deaths in this age group worldwide. The most frequent country of origin in our sample was Equatorial Guinea (68.5%), as has been often the case in previous case series.4–7
We should also highlight that only 5.6% have completed prophylaxis, which was consistent with previous studies conducted in Spain and elsewhere in Europe. This may have to do with the perceived low risk of contracting the disease or with having taken medication that was not appropriate for the specific travel destination.4–9
Furthermore, it is extremely important to insist on the need to complete prophylaxis as prescribed, as travellers very often drop the treatment on arriving back to Spain.
When it came to the clinical features, the initial symptoms were nonspecific in most patients (headache, nausea, vomiting, myalgia and abdominal pain), which contributed to a delayed diagnosis with the subsequent impact on disease outcomes. However, nearly 96% of patients had fever at the time of diagnosis, a finding that was consistent with those of other studies in Europe,10 and therefore the differential diagnosis of febrile patients that had recently travelled to endemic countries should always include malaria. It is important to take into account that in children with immune disorders, absence of fever does not rule out the disease.
Gastrointestinal symptoms were frequent and associated with all involved species, contrary to the evidence in adults. Respiratory symptoms were less frequent, and their incidence was similar for all Plasmodium species. This fact must not be overlooked, as in children with malaria, bronchitis and its complications are a common occurrence and often hinder correct diagnosis because they are misinterpreted as manifestations of respiratory infections.11 Involvement of the lungs, pulmonary oedema and acute respiratory distress are more frequent in cases of severe malaria caused by P. falciparum. The one child infected by P. malariae presented with isolated respiratory symptoms, without fever or any other manifestations, probably due to the characteristic chronic asymptomatic parasitaemia observed in children infected by this species.
Thus, since malaria in children manifests with highly nonspecific symptoms that overlap with those of other infectious and non-infectious diseases, malaria should be suspected in any child with fever coming from an endemic region.12
The classic clinical picture of a malaria attack can guide the correct diagnosis (high fever, chills, severe headache), but does not occur in every case and still requires microbiological confirmation. Other manifestations that may be present include pallor of skin and mucosae, hepatomegaly and splenomegaly.
In the physical examination, independently of the species involved, hepatomegaly was one of the most frequent findings (>50%), especially in parasitic infections by P. falciparum, as the RBCs infected by this species form surface protrusions (knobs) that facilitate their adhesion to endothelial receptors of the liver sinusoids and obstruct capillary blood flow as a result of microvascular damage.13,14 Other, less specific mechanisms associated with this species are generation of hydroxyl radicals and cholestasis secondary to changes in bile secretion, which can also account for the characteristic hepatic manifestations.14,15
Our patients developed splenomegaly in most of the episodes caused by P. falciparum, P. ovale and P. vivax. In infections with P. vivax and P. ovale, enlargement of the spleen is associated with milder fever, whereas in case of infection with P. falciparum the clinical manifestations include high fever and hepatomegaly. Independently of the involved species, splenomegaly is associated with an abnormal immune response with massive deposition of immune complexes due to hyperproduction of polyclonal IgM by B lymphocytes.12,16
When it came to laboratory tests, the most important findings were haemolytic anaemia, thrombocytopenia and elevation of lactate dehydrogenase and indirect bilirubin. Anaemia, defined as a haemoglobin concentration of less than 11g/dL, was the most frequently laboratory finding, in most cases of the haemolytic form and associated with anisocytosis and reticulocytosis. Contrary to adult patients (in whom thrombocytopenia is more common), anaemia is one of the most frequent complications in children with malaria, especially from age 1 to 3 years, and is associated with a high mortality, as is also the case in pregnant women. Anaemia in patients with malaria results from the extravascular haemolysis caused by activation of splenic and other macrophages for phagocytosis of RBCs, infected and uninfected alike, as extrinsic and intrinsic changes in these cells enhance their recognition and phagocytosis.8,9,17
In our patients, thrombocytopenia only occurred in episodes caused by P. falciparum in isolation or in mixed infection, infections in which this is usually the most frequent abnormality detected in the complete blood count in both children and adults, in up to 85% of cases. Elevation of acute phase reactants was also frequent, most commonly of C-reactive protein, the erythrocyte sedimentation rate and lactate dehydrogenase, which was consistent with previous studies.18,19
At this point, we ought to remember that malaria is a disease caused by the Plasmodium parasite and transmitted through the bite of the female Anopheles mosquito. The literature also describes vertical transmission from mother to foetus and transmission through organ transplantation, blood transfusion and accidental puncture with contaminated sharps. Traditionally, 4 species of Plasmodium were known to affect humans: P. falciparum, P. vivax, P. ovale and P. malariae. Recent evidence has shown that P. knowlesi, originally known to infect nonhuman primates, can also infect humans, especially in the region of Malaysia and Borneo.
When it came to the laboratory methods used for parasitological diagnosis, light microscopy examination of peripheral blood smears with Giemsa stain was performed and positive in 100% of cases. This test should be included in the evaluation of every patient with suspected malaria, as it allows a rapid and inexpensive diagnosis with a high sensitivity (92–98%) and specificity (85–99%). It also allows differentiation of the involved species of Plasmodium, as the morphological abnormalities that occur in red blood cells vary depending on the species. However, there are limitations to this technique, as it requires qualified staff and can lead to underdiagnosis of cases with low or mixed parasitaemia. In nearly 50% of patients, detection of Plasmodium antigen by flow chromatographic immunoassay was also performed, which was positive in 100% of cases.
Detection by means of nucleic acid amplification techniques was used in only 8 patients (15%), as this method was introduced in our laboratory in 2011, and was positive in every case. The advantage of using molecular techniques is that they are more sensitive than conventional methods and are therefore very useful in cases with low parasitaemia where direct examination may be negative. However, in this sense it is important to take into account the bias of diagnostic confirmation: cases where there is a stronger clinical suspicion are the ones most frequently assessed with the recommended techniques, resulting in overrepresentation of positive tests and overestimation of the sensitivity of the test in question. Different techniques should be used in each case to establish a more accurate diagnosis and avoid potential biases.20
In our study, the predominant species was P. falciparum. This finding reflects the prevalent distribution of this species in the African continent, where most of our patients were from or had travelled to. In other areas, such as South America, P. vivax gains in importance. The severity criterion found most frequently was hyperparasitaemia (>5% or >50000trophozoites/L), which was consistent with the results of other studies conducted in Spain.4,5,21
As for treatment, we ought to highlight that according to the latest reports published by the Word Health Organization in 2018 and the treatment protocols of renowned infectious disease units in Spain, it is believed that in patients with uncomplicated malaria, in good general health and that tolerate medication well, treatment can be delivered on an outpatient basis with follow-up 12–24h later. In addition, the latest guidelines of the Sociedad Española de Infectología (Spanish Society of Infectious Disease) hospital admission is not a must in these patients, although hospitalisation needs to be considered in patients that may develop complications or with poor oral tolerance.22–25
Prescribing treatment requires knowledge of the species of Plasmodium involved as well as the region of origin of the child to assess resistance to antimalarial drugs and thus achieve an optimal response to treatment. The primary goal of treatment is to achieve rapid and complete elimination of the parasite from the blood of the patient to prevent uncomplicated malaria from progressing to severe disease, death or chronic infection.
Artemisinin-based combination therapy is currently the cornerstone of current treatment guidelines for malaria due to P. falciparum and, since it is unlikely that alternative drugs to artemisinin derivatives will be authorised for at least a few years, preserving their efficacy by preventing the emergence of resistance is of the essence. Infection with P. vivax should be treated with chloroquine in regions where this drug continues to be effective. In order to reduce transmission, the antimalarial regimen should include a single low dose of primaquine.
In our case series, the medication used most frequently was quinine combined with sulfadoxine–pyrimethamine, which can be explained by the retrospective nature of the study, as many patients were treated years ago before the development of the antimalarial drugs currently considered the first-line treatment. The mean duration of treatment was 8 days (similar to previous reports in the medical literature). The response to treatment was good in all cases in our study, although it varies in the literature between studies.21,24–28
Despite all the advances made in the diagnosis and treatment of malaria, we must not forget the importance of preventive measures (specific chemoprophylaxis, vector control strategies and specific vaccines currently under investigation), which have a significant impact on the control of this disease.2,28
ConclusionsMalaria continues to be present in our region as a consequence of immigration and travel to endemic countries. It should always be suspected in febrile children originating from an endemic region or that have travelled to affected areas in the past year. In general, there is a low awareness of the risk of having malaria following travel to an endemic region, which results in a low prophylaxis coverage. Patients that receive appropriate specific treatment exhibit high cure rates, as reflected in our study.
Conflict of interestThe authors have no conflicts of interest to declare.
We thank the Department of Paediatrics and the Department of Admissions of the HUP La Fe for their help in the appropriate collection of data for the study. We thank Dr. María Angeles Delás González, Associate Professor of the UCH-CEU, Hospital Castellón, Valencian Community, for her contributions to the project.
Please cite this article as: Ramirez JH, Urtasun A, Roselló M, Garrido M, Peman J, Otero MC. Estudio descriptivo de los casos de malaria en la población pediátrica en un hospital de referencia de Valencia, España, entre 1993 y 2015. An Pediatr (Barc). 2020;92:21–27.