Infection by cytomegalovirus (CMV) is one of the most common congenital infections, with a global prevalence of 0.3%–2.4%. In Spain, CMV screening is not performed during pregnancy, but rather in neonates with risk factors, and, in many hospitals, in those born small for gestational age (SGA). Screening is usually performed by measurement of the viral load in urine by polymerase chain reaction (PCR) and/or head ultrasound in search of compatible features.
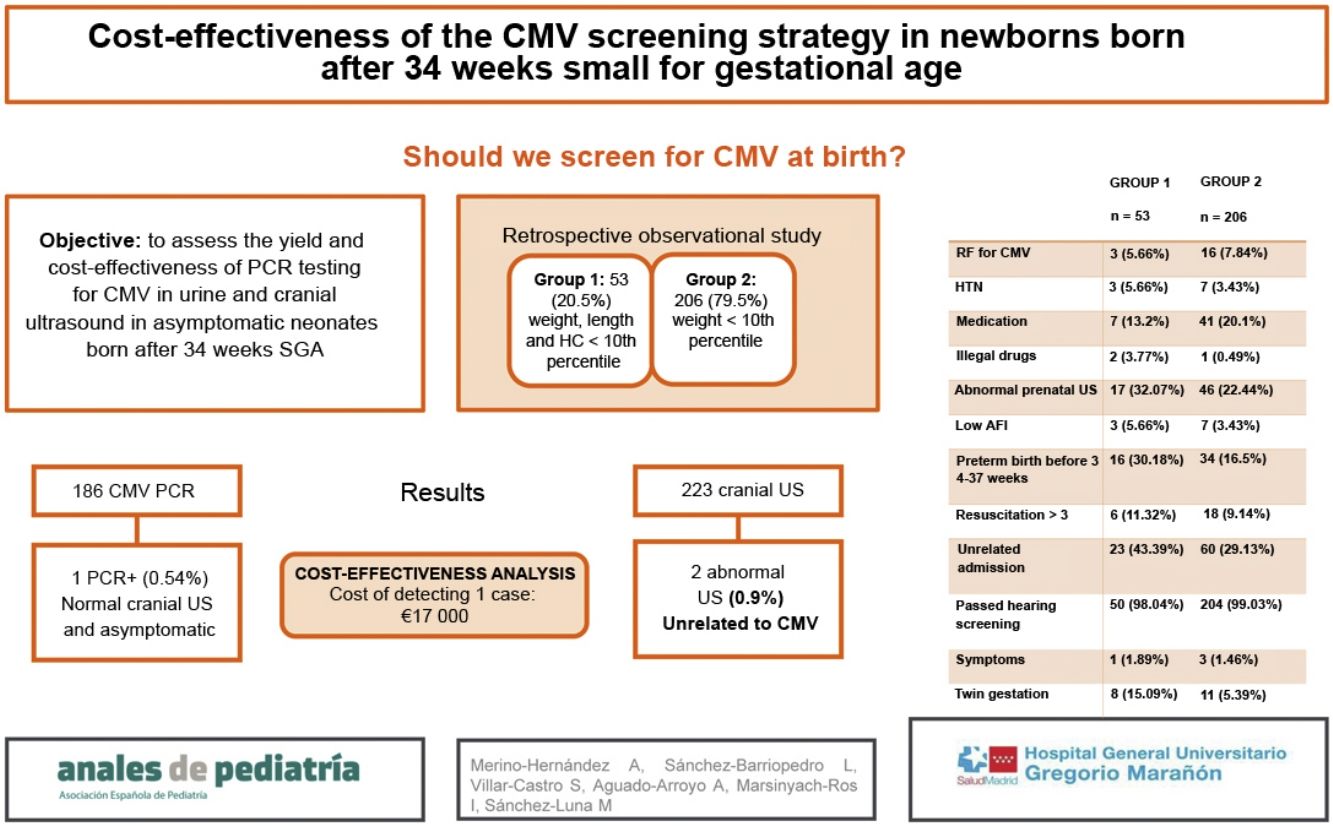
The aim of the study was to assess the yield of the CMV PCR test in urine and head ultrasound examination in asymptomatic neonates born SGA after 34 weeks’ gestation. The secondary objective was to assess the cost-effectiveness of this strategy.
Design and methodsWe conducted an observational and retrospective study between January and December 2019 in a tertiary care hospital. It included neonates delivered after 34 weeks, SGA and without additional risk factors assessed with a CMV PCR test in urine and/or head ultrasound.
ResultsThe sample included 259 patients. It was divided in 2 groups: group 1, patients with a head circumference, weight and length below the 10th percentile (53 patients; 20.5%), and group 2, patients in whom only the weight was below the 10th percentile (206 patients; 79.5%). The incidence of late preterm birth, twin pregnancy, neonatal admission and exposure to illicit drugs during gestation was higher in group 1. A total of 186 urine PCR tests and 223 head ultrasounds were performed overall, and both tests were performed more frequently in group 1 (P=.002). There was only 1 positive CMV PCR test result in the sample (0.54%), corresponding to a patient in group 2 with no abnormal sonographic findings who remained asymptomatic throughout the follow-up. Two head ultrasound examinations yielded abnormal findings, in both cases unrelated to congenital CMV infection. We performed a cost-effectiveness analysis and determined that the cumulative cost of head ultrasound examinations and urine CMV PCR tests in our sample amounted to Є17 000 for the detection of a single asymptomatic positive case.
ConclusionIn our population, screening for congenital CMV infection in asymptomatic late preterm and term newborns whose only risk factor is SGA does not seem to be cost effective. It would be necessary to expand the sample to other populations.
El citomegalovirus (CMV) es una de las infecciones congénitas más frecuentes, con una prevalencia del 0.3–2.4%. En España, al no formar parte del cribado gestacional, se realiza screening de los recién nacidos con factores de riesgo, y, en muchos centros, de los que presentan bajo peso para la edad gestacional (BPEG). Para ello se realiza, generalmente, determinación de PCR (Polymerase Chain Reaction) del virus en orina y/o ecografía transfontanelar en busca de imágenes compatibles.
El objetivo del estudio es evaluar el rendimiento de la PCR de CMV en orina y ecografía transfontanelar, en recién nacidos > 34 semanas asintomáticos, sin factores de riesgo, con BPEG. El objetivo secundario es evaluar el coste-efectividad.
Material y métodosEstudio observacional, descriptivo y retrospectivo, entre enero y diciembre de 2019, en un hospital de tercer nivel (IIIC). Incluye recién nacidos >34 semanas, sin factores de riesgo con BPEG, con PCR de citomegalovirus en orina y/o ecografía transfontanelar realizada.
ResultadosSe incluyeron en el estudio 259 pacientes. Se dividió la muestra en grupo 1 (pacientes con percentil menor de 10 para talla, peso y perimétro cefálico (53 pacientes, 20.5%)) y grupo 2 (percentil menor de 10 para peso (206 pacientes, 79,5%)). En el grupo 1 se objetivó mayor incidencia de prematuridad tardía, embarazo gemelar, ingreso y consumo de drogas ilegales durante el embarazo. Se realizaron 186 PCR de citomegalovirus en orina y 223 ecografías transfontanelares, siendo más frecuente la realización de ambas pruebas en el grupo 1 (p=0.002). Se obtuvo un único resultado positivo en orina en el total de la muestra (0.54%) (grupo 2), sin alteraciones en la ecografía y sin sintomatología a lo largo de su evolución. Se encontraron 2 ecografías transfontanelares alteradas con hallazgos no relacionados con infección congénita por CMV. Se realizó un estudio de coste-efectividad, en el cual el coste del total de ecografías transfontanelares y CMV en orina realizadas en nuestra muestra ascendió a 17000Є, para detección de un caso positivo asintomático.
ConclusionesEn nuestra población no parece coste efectivo realizar pruebas de cribado de infección congénita por CMV a recién nacidos a término y casi a término asintomáticos, cuyo único factor de riesgo es BPEG. Sería necesario ampliar la muestra a otras poblaciones.
Congenital infection by cytomegalovirus (CMV) is among the most important congenital infections in terms of the morbidity it causes in affected newborns. In the general population, it frequency ranges from 0.3%–2.4%.1 It is in the group of congenital diseases known as the TORCH infections (toxoplasmosis, rubella, CMV and syphilis, among others)2 that share similar characteristics, chief of which are transmission through the placenta or through direct contact during or after delivery, the tendency to go undetected during pregnancy, diagnosis through serology or molecular techniques (polymerase chain reaction [PCR]) and a similar clinical presentation.
Screening programmes in pregnant women and ongoing research in the field have managed to significantly reduce the incidence of congenital infections in recent years. At present, there is a tendency to perform postnatal assessments of newborns with risk factors for some of the congenital infections that are not included in the routine newborn screening programme, such as CMV. In this context, it has been several years since postnatal screening techniques for CMV have been established for diagnosis of this congenital infection in newborns.
For years, efforts have been made to establish the association of low birth weight with neurodevelopmental abnormalities later in life. However, Lohaugen et al.3 evinced that low birth weight in isolation did not increase the probability of impaired cognitive function, with the probability increasing when it was associated with other risk factors, such as microcephaly. Still, TORCH screening strategies have been implemented for years in this group of infants assuming an association with low birth weight. In opposition to this, Krishnamurthy et al.4 found that low birth weight as an isolated risk factor did not warrant performance of screening in newborns, as results were not significantly different in the comparison of congenital infections in this risk group versus the general population.
Previous studies have attempted to evince an association between abnormalities in prenatal ultrasound examinations5 and the classification of foetuses as having intrauterine growth restriction (IUGR) type 1 or 2 in relation to the eligibility for CMV screening, finding a similarly low risk in both groups, without significant differences between them.6
Notwithstanding, many facilities continue to screen low birth weight newborns for CMV infection. Some of the factors that probably contribute to this practice is the high prevalence of CMV infection in the general population and its silent course in a majority of cases, which makes it difficult to diagnose in pregnant women. Screening in newborns is usually performed by collecting a urine sample at birth to carry out a PCR test for detection of the virus and/or performance of a cranial ultrasound to assess for lesions compatible with congenital CMV infection. Other methods are available, such as serology testing in blood samples, but they are not commonly used for screening on account of their invasiveness.
Due to the lack of stringent written protocols, decisions regarding screening usually rest with the clinician in charge. In addition to the emotional burden placed on families by the performance of these tests, these screening strategies are quite costly for the health care system, as there is a large number of newborns with low birth weight as the sole risk factor, resulting in a considerable number of screening tests. Yet, screening continues to be a customary practice in many facilities.
The primary objective of the study was to assess the clinical yield of ordering a PCR test for detection of CMV in urine and transfontanellar ultrasound in newborns born at term or near term whose only risk factor for congenital CMV infection is low birth weight for gestational age. The secondary objective was to determine whether this was a cost-effective strategy.
Material and methodsStudy designWe conducted a retrospective, observational and descriptive study in the department of neonatology of the Hospital Universitario Gregorio Marañón de Madrid (HGUGM) between January and December 2019. This non-interventional observational study was approved by the ethics Committee of the hospital (code: CMVBAJO 1.0).
SampleThe study included newborns delivered at or after 34 weeks of gestation that underwent a urine test for detection of CMV and/or a transfontanellar ultrasound examination due to low birth weight for gestational age during the study period.
The HGUGM is a tertiary care hospital (level IIIC)7 managing an average of about 4500–5000 births a year. The exclusion criteria were: presence of chromosomal disorder or severe malformation, birth before 34 weeks of gestation need of hospital admission at birth due to morbidity associated with low birth weight.
Data collectionWe collected epidemiological and clinical data on newborns who did not require admission at birth and newborns in whom tests were ordered to screen for congenital infection on account of low birth weight: urine polymerase chain reaction test for CMV and/or transfontanellar ultrasound. A telephone appointment was scheduled to give the results of these tests after the patients had been discharged from the maternity ward.
We created a database to record data for different variables collected from the inpatient care records of the patients: maternal age, risk factors for CMV infection (siblings younger than 3 years, maternal risk contact, maternal HIV infection or compatible findings in prenatal ultrasound), maternal gestational or pregestational hypertension (HTN), maternal use of medication or drugs during pregnancy, abnormal findings in prenatal ultrasound, gestational age at birth, date of birth, infant sex, need of neonatal resuscitation, arterial cord blood pH, 1- and 5-minute Apgar scores, need of neonatal admission, hearing screening, birth weight, length and head circumference and their respective percentiles, manifestations compatible with CMV (hepatosplenomegaly, petechiae, chorioretinitis, hearing loss…) and the results of the CMV urine PCR test and findings of transfontanellar ultrasound, if performed.
Unit protocolIn our unit, congenital CMV screening is performed routinely in newborns small for gestational age, defined as a birth weight below the 10th percentile (P10).8 The percentiles were calculated using the INTERGROWTH tables as reference. The same tables were used for twin and singleton pregnancies.
Two diagnostic tests were available, the performance of which depended exclusively on the judgment of the physician in charge. One consists in placing a urine collection bag to then perform a PCR test for detection of CMV, with titres of less than 150 copies/mL considered a negative result. If the result was positive, a blood sample was collected from the newborn to perform confirmatory PCR and serology tests. Quantitative PCR tests for CMV in urine were performed with the Abbott RealTime CMV assay, with a cost of Є18 (plus VAT) per test.
The indication for transfontanellar ultrasound at birth in our unit was low birth weight (in addition to other indications that are out of the scope of the study). This test was performed by paediatric radiologists with extensive experience in neonatal sonography using a Samsung RS80A machine with a CF4−9 convex probe and a L3−12 linear probe, with an approximate cost of Є60 per ultrasound examination. The examination was performed through the anterior fontanelle with state-of-the-art technique, scanning standard sections including 6 coronal planes (prefrontal, hypothalamic sulcus, ventral lateral nucleus of the thalamus, pulvinar, atrium and parieto-occipital sulcus) and 5 sagittal planes (midsagittal plane, caudothalamic groove and insular region of each side), and using a high-frequency transducer to examine the parenchyma.9
If either test turned out positive, a more extensive evaluation was performed, confirming the results with a new urine test, a blood PCR test and imaging. In addition, while the newborn hearing screening was not repeated, the infant was referred to the otorhinolaryngology department for follow-up.
Statistical analysisWe used the Shapiro–Wilk test to assess whether quantitative variables followed a normal distribution in the sample, and found that they did not. Therefore, we expressed the results for quantitative study variables in each patient as median and interquartile range (IQR). The results for categorical variables were summarised as absolute frequencies and percentages. To compare quantitative data, we used the Mann–Whitney U test, and to compare categorical data, the χ2 test. The statistical analysis was performed with the software Stata, version 14.0. We considered results statistically significant if the P-value was < 0.05.
ResultsBetween January and December 2019, we included 259 patients in the study. The sample was divided in 2 groups. Group 1 included newborns with a birth weight, length and head circumference below the 10th percentile.10 Group 2 included newborns with a birth weight below the 10th percentile but with a head circumference and length above the 10th percentile. Group 1 included 53 newborns (20.5%) and group 2, 206 (79.5%), and we made a descriptive analysis of the risk factors associated with infection by CMV.
In group 1, we found a higher frequency of late preterm birth (.02), twin pregnancy (.01), admission unrelated to congenital CMV (.047) and maternal illicit substance use during pregnancy (.04), without significant differences in the rest of the variables (Table 1). In addition, the mean gestational age was lower in this group (Table 2), in part related to 10 of the 53 deliveries (18.9%) in group 1 being induced due to IUGR and 7 (13.2%) due to nonreassuring foetal status based on cardiotocographic features.
Comparison of the groups.
| Group 1 n=53 | Group 2 n=206 | Total N=259 | P | |
|---|---|---|---|---|
| RF for CMV; n (%) | 3 (5.66%) | 16 (7.84%) | 19 (7.42%) | .61 |
| HTN; n (%) | 3 (5.66%) | 7 (3.43%) | 10 (3.91%) | .43 |
| Medication; n (%) | 7 (13.2%) | 41 (20.1%) | 48 (18.75%) | .27 |
| Illegal drugs; n (%) | 2 (3.77%) | 1 (0.49%) | 3 (1.17%) | .04 |
| Abnormal prenatal US; n (%) | 17 (32.07%) | 46 (22.44%) | 63 (24.51%) | .12 |
| Low AFI; n (%) | 3 (5.66%) | 7 (3.43%) | 10 (3.91%) | .43 |
| Preterm birth before 34−37 weeks; n (%) | 16 (30.18%) | 34 (16.5%) | 50 (19.31%) | .02 |
| Resuscitation > 3; n (%) | 6 (11.32%) | 18 (9.14%) | 24 (9.27%) | .58 |
| Unrelated admission; n (%) | 23 (43.39%) | 60 (29.13%) | 83 (32.05%) | .047 |
| Passed hearing screening; n (%) | 50 (98.04%) | 204 (99.03%) | 254 (98.83%) | .55 |
| Symptoms; n (%) | 1 (1.89%) | 3 (1.46%) | 4 (1.54%) | .82 |
| Twin gestation; n (%) | 8 (15.09%) | 11 (5.39%) | 19 (7.39%) | .01 |
AFI, amniotic fluid index; CMV, cytomegalovirus; HTN, hypertension; RF, risk factor; US, ultrasound.
*Comparison of small for gestational age groups. Group 1: weight, length and head circumference < 10th percentile. Group 2: birth weight < 10th percentile.
Comparison of groups.
| Group 1 n=53 | Group 2 n=206 | Total | P | |
|---|---|---|---|---|
| Maternal age (years); median (IQR) | 34 (32−37) | 34 (31−37) | 34 (29−39) | .82 |
| Gestational age (weeks); median (IQR) | 37 (36−39) | 38 (37−40) | 38 (36−40) | .03 |
| Cord blood pH; median (IQR) | 7.27 (7.21−7.32) | 7.25 (7.19−7.3) | 7.25 (7.14−7.36) | .13 |
| 1-min Apgar; median (IQR) | 9 (8−9) | 9 (9−9) | 9 (8−10) | .07 |
| 5-min Apgar; median (IQR) | 10 (9−10) | 10 (9−10) | 10 (9−10) | .03 |
| Weight (g); median (IQR) | 2150 (1.880−2.420) | 2.470 (2.220−2.640) | 2.420 (1.940−2.900) | ≤.01 |
IQR, interquartile range.
*Comparison of small for gestational age groups. Group 1: weight, length and head circumference < 10th percentile. Group 2: birth weight < 10th percentile.
*We did not find an explanation for the statistically significant difference in the 5-minute Apgar score.
A total of 186 CMV urine PCR tests and 214 transfontanellar ultrasound exams were performed in the sample under study, and both tests were performed significantly more frequently in group 1 compared to group 2 (.002).
In group 1, none of the urine PCR tests were positive for CMV. In group 2, only 1 patient had a positive result with 704 058 copies/mL, amounting to 0.54% of the total subset of patients in whom a urine PCR test was performed. This patient had a history of IUGR diagnosed antenatally by Doppler ultrasound. The postnatal transfontanellar ultrasound did not detect any abnormalities and the patient received a diagnosis of asymptomatic congenital CMV infection after the positive urine PCR test. The patient underwent a follow-up magnetic resonance scan at 3 weeks post birth that evinced mild bilateral alteration of signal intensity in the periatrial region and, less noticeably, the frontal region, with no clear association with white matter involvement due to CMV infection, leading to recommendation of an additional magnetic resonance scan that was not performed in the end, as the patient remained asymptomatic in the follow-up and the hearing test was normal. The patient was followed up in the infectious disease and otorhinolaryngology departments and did not require antiviral treatment.
The transfontanellar ultrasound found abnormalities in 2 cases, both in group 2, amounting to 0.93% of the total ultrasound examinations performed. The alterations were chance findings, subsequently confirmed by magnetic resonance, unrelated to congenital CMV infection: an ischaemic lesion in the caudate nucleus and right cerebellar hypoplasia and bleeding.
We performed a cost-effectiveness analysis and found that the cost of screening with transfontanellar ultrasound and CMV testing in urine amounted to approximately Є17 000 in total for the detection of a single case of congenital CMV infection, in this case, an asymptomatic one. Due to the absence of symptomatic cases, we were unable to calculate the number needed to treat. If newborns with risk factors related to prenatal low weight are not taken into account, the cost would decrease to Є15 800. If the performed transfontanellar ultrasound examinations are not taken into account, the cost of screening only with the CMV urine PCR test would be Є4050 for the detection of a single case.
DiscussionWe found a low frequency of positive CMV screening tests in asymptomatic term and near term neonates in our sample (0.54%), which was consistent with similar studies that have found a prevalence ranging from 0.2% to 2.5%.1,11
Despite its low prevalence, CMV continues to be the most frequent cause of congenital viral infection in Spain. It can cause important sequelae, such as hearing loss, intellectual disability, cerebral palsy, seizures and learning disabilities, which may occur in up to 20% of infected infants.12 Congenital CMV infection is the leading non-inherited cause of sensorineural hearing loss. The incidence and severity of these sequelae may decrease with early treatment of newborns with congenital CMV infection, which is the reason that some authors, such as Rawlinson et al.,13 have suggested the possibility of universal screening, a strategy that Gantt et al. advocate for, as they consider it cost-effective.14 However, many other cost-effectiveness studies do not support universal screening on account of its low yield and high cost, also given that the same authors do not recommend treatment of asymptomatic newborns.2,15
At present, PCR testing of urine for detection of CMV is one of the most widely used screening methods because it is non-invasive and has exhibited a high sensitivity.16,17 Other screening methods have been proposed in recent studies, such as PCR testing in saliva samples, on account of the ease of sample collection,18 and PCR testing of the dry blood spot sample collected for newborn screening of metabolic and endocrine diseases, on account of its potential low cost and high yield.19
In our sample, the only patient that had a positive CMV urine PCR test did not receive any treatment and was followed up in the paediatric infectious disease unit, with resolution of the infection without complications, which is the usual outcome. Similarly, a study by Baquero Artiago et al. found that only 13% of asymptomatic newborns with a positive PCR test developed symptoms during the follow-up.11
Another widely used screening strategy is performance of a transfontanellar ultrasound in low birth weight newborns to assess for abnormalities characteristic of congenital CMV infection, such as intracranial calcifications, ventriculomegaly, cortical atrophy or cystic lesions in the germinal matrix.8 However, some authors argue that these findings are not very specific to congenital CMV infection.4,20,21 In our study, the prevalence of abnormal findings in the ultrasound examination was 0.93%; however, most of these abnormalities could be considered chance findings unrelated to CMV infection. The performance of transfontanellar ultrasound as a CMV screening strategy in asymptomatic low birth weight infants, based on our data and the findings of similar studies, does not seem cost-effective.11
Low birth weight has been traditionally associated with congenital infection, but other risk factors for IURG are identified in up to 40% of these infants (maternal HTN, maternal smoking, twin gestation…) that could justify low birth weight. In the study by Vaundry et al., the prevalence of congenital CMV infection in the subset of low birth weight newborns did not differ from the prevalence in the general population.22
Some studies have found an association between symmetrical IUGR and congenital CMV infection.23 However, a study by Espiritu et al. found a low risk of congenital CMV infection in both the symmetrical IUGR and the asymmetrical IUGR groups, without significant differences between groups.6 In our sample, out of the 186 newborns screened with the urine PCR test, only 1 had a positive result, a patient that belonged to group 2 (low birth weight only).
In the period under study, a total of, 186 CMV urine PCR tests and 214 transfontanellar were performed. This amounts to a total cost of Є17 000 for diagnosis of a single case. To this cost, we must add the anxiety and worry elicited in the family by the performance of diagnostic tests for the detection of a disease with a low prevalence.6 Based on these findings, screening of asymptomatic newborns whose only risk factor is low birth weight could be considered, in specific settings and populations with a low prevalence, a strategy that is not cost-effective.24
Until recently, due to the lack of effective treatment for congenital CMV infection acquired during gestation that would change patient outcomes, the implementation of universal screening of CMV infection in pregnant women did not seem to be cost-effective. However, recent studies25 have found encouraging results for the efficacy of valaciclovir given to pregnant women with primary infection in reducing the incidence of congenital CMV infection in the offspring.26 In the future, these outcomes could warrant contemplating universal screening of pregnant women for early detection and possible treatment of congenital CMV infections. The introduction of screening during pregnancy, combined with the treatment of pregnant women with primary infection and the low incidence of congenital CMV infection in low birth weight infants, may be reasons not to routinely screen for congenital CMV infection in this subset of patients.
There are several limitations to our study, such as its retrospective design; since the newborns were selected from those who attended the newborn evaluation in the neonatal clinic, we may have missed information, a drawback that we tried to palliate through the exhaustive review of the health records of mothers and infants and diagnostic tests records. In addition, there may have been false negative results in the CMV urine PCR test.
ConclusionIn conclusion, in our population, routine performance of tests for screening of congenital CMV infection does not appear to be cost effective in asymptomatic term and near-term newborn infants whose only risk factor is low birth weight, especially screening by means of transfontanellar ultrasound. The performance of the PCR test in urine could be reserved for patients with low birth weight and no associated risk factors for this status (twin gestation, maternal hypertension, etc). Transfontanellar ultrasound does not seem to be indicated in this context. However, to generalise these results, further research should be conducted in other populations.