There have been significant changes in community acquired pneumonia (CAP) in children in the last decade. These changes are related to epidemiology and clinical presentation. Resistance to antibiotics is also a changing issue. These all have to be considered when treating Community acquired pneumonia (CAP). In this document, two of the main Spanish pediatric societies involved in the treatment of CAP in children, propose a consensus concerning therapeutic approach. These societies are the Spanish Society of Paediatric Infectious Diseases and the Spanish Society of Paediatric Chest Diseases. The Advisory Committee on Vaccines of the Spanish Association of Paediatrics (CAV-AEP) has also been involved in the prevention of CAP. An attempt is made to provide up-to-date guidelines to all paediatricians. The first part of the statement presents the approach to ambulatory, previously healthy children. We also review the prevention with currently available vaccines. In a second part, special situations and complicated forms will be addressed.
La neumonía adquirida en la comunidad (NAC) en la edad pediátrica ha sufrido, en la última década, una serie de cambios epidemiológicos, clínicos, etiológicos y de resistencias a antibióticos, que obligan a replantear su abordaje terapéutico. En este documento, dos de las principales sociedades de especialidades pediátricas involucradas en el diagnóstico y tratamiento de esta entidad, como son la Sociedad Española de Infectología Pediátrica y la Sociedad Española de Neumología Pediátrica, así como el Comité Asesor de Vacunas de la AEP, proponen unas pautas consensuadas de tratamiento y prevención, con el fin de proporcionar a todos los pediatras una guía actualizada. En esta primera parte del consenso, se aborda el tratamiento de los pacientes sin enfermedades de base relevantes con NAC que no precisan ingreso hospitalario, así como la prevención global de esta patología con vacunas. En un siguiente documento se expondrá el abordaje terapéutico tanto de aquellos pacientes en situaciones especiales como de las formas complicadas de la enfermedad.
Community acquired pneumonia (CAP) is the single main cause of child mortality worldwide. It is estimated to be responsible for 1.2 annual million deaths in children under 5 years of age, which accounts for 18% of all deaths at this age, 99% of them in developing countries.1
In developed countries and regions, such as North America, Europe, Oceania and Japan, there are estimated to be up to 2.6 million annual cases of CAP in children under 5 years of age, causing 1.5 million hospitalisations and, approximately, 3000 deaths,2 more than the number of deaths for meningitis.
In the last decade, the aetiology, clinical presentation and evolution of CAP in the paediatric population have changed significantly with the introduction of vaccines against pathogens involved in its aetiology (such as Haemophilus influenzae [H. influenzae] type b and Streptococcus pneumoniae [S. pneumoniae]), the better use of antibiotics, as well as other factors which have not yet been explained but are probably associated with independent epidemiological trends.
As already explained in the document on the aetiology and diagnosis of CAP agreed by these two paediatric associations,3 the main aetiological agents are viruses and S. pneumoniae. Viruses affect mainly children under 4–5 years of age, while S. pneumoniae affects children of any age. However, in the last 10–15 years the incidence of complicated pneumonias, manifesting as either pleural effusion or necrotising pneumonia, has steadily increased. Changes have also been observed in the age of onset of complicated pneumonias. Where previously it was more frequent in children under the age of 2–3 years, it now predominates in children 2–5 years of age. There has also been a slight increase in the number of cases caused by Staphylococcus aureus (S. aureus), some caused by strains produced by certain virus factors which make them more serious.
Paediatricians treating children with CAP4 have access to a huge range of therapies, and in many countries clinical guidelines are not followed.5,6 Therefore, one of the most ambitious goals of this consensus is to harmonise therapeutic measures against this disease in Spain and improve control measures.7 In this document, based on the scientific information available and the experience of the authors, initial measures are proposed which we believe are more adequate for the therapeutic treatment of CAP. Also, the prevention measures available against CAP in the population of children are summarised. In another document, soon to be published in this journal the therapeutic approach to complicated cases and special circumstances will be presented.
Current status of resistances to antimicrobial drugsDrug-resistant bacteria that can potentially cause CAP include S. pneumoniae, S. aureus, Streptococcus pyogenes (S. pyogenes) and H. influenzae type b. In Spain, other causal agents of CAP, such as Mycoplasma pneumoniae (M. pneumoniae) or Chlamydophila pneumoniae (C. pneumoniae), or viruses, are not drug-resistant. M. pneumoniae and C. pneumoniae are usually sensitive to macrolides and the only virus to be treated with antiviral drugs, the flu virus, so far does not present resistance to oseltamivir in our area.
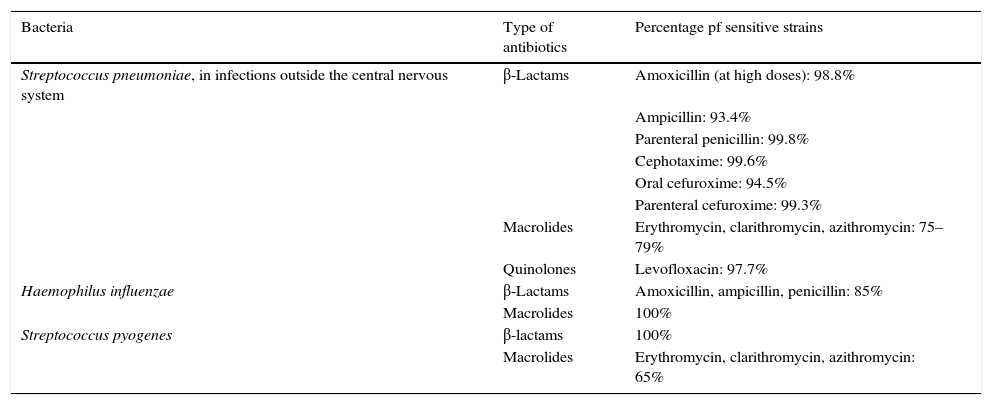
The most reliable data on the drug-resistance of the main respiratory pathogens in our area are periodically provided by the multi-centre study known as Sensitivity to Antibiotic Drugs Used in the Community in Spain (SAUCE in Spanish). The latest, published in 2010 as the SAUCE-4 study,8 offers results on sensitivity and resistance according to official cutoff points (CLSI cutoff points). It contains a total of 2559 isolations of S. pneumoniae, 2287 of S. pyogenes and 2287 of H. influenzae, and these are compared to those recorded in the previous 11 years. In summary, the most relevant data are described in Table 1. For S. pneumoniae, as regards sensitivity to β-lactams, currently almost all strains circulating in Spain are sensitive to oral amoxicillin and intravenous penicillin and ampicillin, and also cefuroxime, if we wish to broaden the spectrum. They are all sensitive to cephotaxime. In recent years, the percentage of penicillin-resistant strains (intermediate sensitivity or total resistance) have risen from 60.0% to 22.9%. The proportion of strains with total resistance to oral penicillin (MIC≥2) has decreased drastically from 36.5% to 0.9%. Also, 0% presents total resistance (MIC≥8) for parenteral penicillin and only 0.2% intermediate sensitivity (MIC≥4). There are still high rates of resistance to macrolides (21–25%).
Sensitivity of the main bacteria causing CAP in Spain (data from the SAUCE-4 study).
| Bacteria | Type of antibiotics | Percentage pf sensitive strains |
|---|---|---|
| Streptococcus pneumoniae, in infections outside the central nervous system | β-Lactams | Amoxicillin (at high doses): 98.8% |
| Ampicillin: 93.4% | ||
| Parenteral penicillin: 99.8% | ||
| Cephotaxime: 99.6% | ||
| Oral cefuroxime: 94.5% | ||
| Parenteral cefuroxime: 99.3% | ||
| Macrolides | Erythromycin, clarithromycin, azithromycin: 75–79% | |
| Quinolones | Levofloxacin: 97.7% | |
| Haemophilus influenzae | β-Lactams | Amoxicillin, ampicillin, penicillin: 85% |
| Macrolides | 100% | |
| Streptococcus pyogenes | β-lactams | 100% |
| Macrolides | Erythromycin, clarithromycin, azithromycin: 65% |
Adapted from Pérez-Trallero et al.8
For H. influenzae, 15.7% are producers of β-lactamases and, therefore, resistant to penicillin, ampicillin or amoxicillin. This percentage has decreased, from the previous level of 25.7%.
These data, based on samples from children and adults taken 6–7 years ago, can be combined with the findings of a recent study by Heracles in the Community of Madrid (May 2011–April 2013),9,10 where 100% of S. pneumoniae strains isolated in children under 15 years old with invasive pneumococci disease outside the central nervous system—including bacteraemia pneumonias and empyema—are sensitive to penicillin and cephotaxime.
Adjuvant support treatmentIn children with CAP, antibiotic therapy at times needs to be complemented with other strategies, although this is less frequent in patients not requiring hospitalisation.
Children with pneumonia usually feel associated pain (pleuritic, abdominal, headache) and discomfort or pain due to inflammation of upper airways (otalgia, odynophagia). Analgesia is recommended for relief, especially in cases of pleuritic pain, because it interferes with coughing and breathing.11,12 Paracetamol can be used (15mg/kg/6h; up to a maximum of 75mg/kg/day) or ibuprofen (5–10mg/kg/6–8h). Fever must be controlled with these same agents, as oxygen requirements increase. There is insufficient evidence that mucolytic and cough suppressants are beneficial, and in theory, medications with codeine or antihistamines should not be used in young children.13
Increased effort of breathing and fever increase the requirement for fluids. The ideal way to provide them is orally, in small amounts and frequently.
Ambulatory treatment of non-complicated CAP with antibioticsIndication for the use of antibioticsEmpirical treatment of CAP is based on the pathogens most frequently involved. However, one of the most important problems is correct distinction between probable viral aetiology and probable bacterial aetiology. Clinicians tend, mistakenly, to use antibiotics in excess, which leads to an increase in antimicrobial resistances. In patients aged less than 2 years, with mild clinical lower airway manifestations and a history of correct immunisation according to age against H. influenzae type b and S. pneumoniae bacterial aetiology is unlikely.14
Antibiotics are indicated in typical CAP where bacterial aetiology is suspected. In cases of atypical CAP they should only be used in children over 4–5 years old and in certain younger patients if the infection is serious.
For treatment under special circumstances (allergy to β-lactams, base disease, immunodepressed, etc.), or patients requiring hospitalisation, more information will be available in the specific document that will be published in a subsequent issue of this journal.
Selection of the antibiotic, route, dosage and durationTypical CAPIf it has been decided to initiate ambulatory antibiotic treatment in typical CAP with no criteria for hospital admission, considering that most are caused by pneumococci and that, currently, almost all are sensitive to penicillin and amoxicillin,8 the antibiotic of choice is 80–90mg/kg/day oral amoxicillin, every 8h (Table 2). This recommendation is consistent with current international guidelines.12,15 The maximum recommended dose, according to the package leaflet, is 2g every 8h, given the good tolerance of this antibiotic.
Ambulatory antibiotic treatment for children with CAP who do not need hospitalisation.
| Typical CAP (with suspected or confirmed aetiology) | ||
|---|---|---|
| Name | Posology | Current duration |
| Oral amoxicillin | 80–90mg/kg/day, divided into 3 doses (every 8h)a | 7 days |
| Atypical CAP with confirmed aetiology or high suspicion of Mycoplasma or Chlamydia. Most used macrolides | ||
|---|---|---|
| Name | Posology | Duration |
| Oral azithromycin | 10mg/kg every 24h (maximum: 500mg/day)b | 3 days |
| Oral clarithromycin | 15mg/kg/day, every 12h (maximum dose: 1g/day) | 7 days |
There may be some controversy regarding the recommended dose. Given the good absorption of this drug and its good penetration at a pulmonary level, as well as the low rates of resistances of S. pneumoniae, doses of 40–50mg/kg/day will suffice in most cases. This consensus recommends, however, higher doses (80–90mg/kg/day) due to the following reasons:16
- –
The use of low doses (40–50mg/kg/day) may lead to reappearance of resistant strains.
- –
Children with respiratory infections frequently vomit, which may cause infradosage scenarios, especially if low doses are used.
- –
In pneumococcal infections of the upper airways (acute otitis media and sinusitis), it is necessary to continue to use high doses due to lower penetration of drugs in these locations. It is preferable to harmonise the dosage of this oral antibiotic for all pneumococcal infections, for the purpose of minimising prescription errors.
The use of clavulanic acid together with amoxicillin in children with typical CAP with no underlying disease and vaccinated against H. influenzae type b, is not justified if there is suspicion of probable pneumococci aetiology, since S. pneumoniae drug-resistance through the production of β-lactamases is still unclear. Furthermore, their use is associated, relatively frequently, with gastrointestinal symptomatology, particularly diarrhoea, which can decrease absorption of amoxicillin.
Macrolides should not be used for the treatment of typical CAP for many reasons, the most important being current resistance of S. pneumoniae to these antibiotics and the risk of bacteraemia in these patients.17 Despite that, they are often incorrectly prescribed for CAP.5
The recommended duration of treatment in a patient with typical CAP with no complications and not requiring admission is 7 days. There are several meta-analyses, based mainly on trials carried out in developing countries, which showed that 3 days of oral amoxicillin will be effective in treating children of 2–59 months of age with CAP that do not require hospitalisation. Although this strategy reduces costs,18,19 it is associated with a high rate of therapeutic failure, and therefore should not be used in Spain.
Atypical CAPIn the case of atypical CAP in children under 4–5 years old, the aetiology is usually viral, and therefore no antibiotics are prescribed. In children over 4–5 years old, in whom M. pneumoniae aetiology is more frequent (up to 40% of CAP in this age group)20 and, to a lesser extent, C. pneumoniae, the use of oral macrolides is recommended,15,20 although there is no clear evidence of its effectiveness in resolving CAP in this population.21,22
The macrolides most used currently (azithromycin and clarithromycin) and their recommended dosage are described in Table 2.15 Erythromycin is clearly not used due to its adverse effects (mainly gastrointestinal) and complicated dosing regimen (every 6h, 10–14 days), which limits its effectiveness.
Evolution and follow-upOnce CAP has been diagnosed and treatment has started a clinical assessment by the paediatrician is recommended after 48h. In non-complicated cases, 90% of patients are afebrile 48–72h after starting antibiotic treatment, and do not need further blood tests or radiological follow-up.3
Only a small proportion need hospital admission. The management of therapeutic failure and the assessment of the hospital admission will be addressed in the second part of this document.
Preventive measures. VaccinesVaccination against certain microorganisms has proven to have an impact on the incidence and mortality of CAP worldwide. The aetiological agents for which there are vaccines available are S. pneumoniae, H. influenzae type b and the flu virus.
Vaccination against S. pneumoniaeThe release of the heptavalent conjugate vaccine led to a global reduction of invasive pneumococcal disease (IPD) in children, given its effect on nasopharyngeal colonisation by the serotypes included in the vaccine and, consequently, in its clinical forms.23 However, incidence of IPD has increased in recent years, mainly complicated CAP especially in children over 2 years,24 produced by serotypes not included in the vaccine.23 In Spain, the most frequent are: 1, 19A, 7F, 3, 6A, 19F. Serotype 1 mainly affects children over 24 months of age and causes, in particular, bacterial pneumonia and pleural empyema. Serotypes 1, 19A and 3 caused 85% of pleural empyema of children in Spain, before the development of new vaccines, according to a study carried out by Laboratorio Español de Referencia de Neumococo del Instituto Carlos III.25 Most serotypes noted are uniformly sensitive to penicillin, except serotype 19A, associated more frequently with resistance, including cephotaxime.
Two conjugated antipneumococcal vaccines are currently authorised in children: decavalent vaccine (VNC10) (Synflorix®, GSK), up to 5 years of age, and tridecavalent (VNC13) (Prevenar 13®, Pfizer), authorised in children up to 17 years of age. Systematic antipneumococcal vaccination is still recommended by the Advisory Committee of Vaccines of the Spanish Paediatric Association in its annual immunisation report.26
13-valent pneumococcal conjugate vaccinePCV13 has the 7 serotypes of the VNC7 and the following 6 additional serotypes: 1, 3, 5, 6A, 7F and 19A, and is approved for the prevention of CAP. Currently, PCV13 offers the widest coverage against pneumococcal disease worldwide,23 including Spain,9,10,25 and therefore, it is recommended for all children under 5 years of age, both healthy and at risk for disease.27
In Madrid, as of July 2012, PCV13 is no longer subsidised, and therefore coverage has decreased to approximately 70%. Still, the data are very good so far, with a decrease in bacterial CAP (87%), pleural pneumococcal empyema (61%) and meningitis (72%), compared with 2007–2010.9
In the United Kingdom, one year after starting vaccination, PCV13 was shown to be effective against additional serotypes (1, 3, 5, 6A, 7F and 19A) in over 50% in children under 2 years of age.28
In France, where there has been systematic vaccination with PCV13 since 2010 (previously with PCV7) recent studies in children under 15 years of age have shown a 16% decrease in CAP overall, and 63% decrease in pneumococcal CAP. This is in addition to a 53% decrease in cases with pleural effusion.29
In the USA, with systematic vaccination with PCV13 since 2010 (previously with PCV7) a 50% overall reduction In IPD has been reported, and a 70% reduction in cases attributed to PCV13, while hospitalisation for CAP in children under 2 years of age fell by 65% in 2012.30,27
In Latin America, several countries have published good results after the introduction of PCV13 in their vaccination calendars, including Argentina, with a 41% reduction in cases of CAP in children under 5 years old.31 In Uruguay, hospitalisation for CAP in the under-14 age group has decreased by 78% overall, and by 92% in cases of pneumococcal origin.32
10-valent pneumococcal conjugated vaccinePCV10 in addition to the serotypes contained in PCV7 incorporates another 3 serotypes: 1, 5 and 7F, and is approved for the prevention of CAP. In a randomised clinical trial (COMPAS study), carried out in approximately 24,000 infants in 3 Latin American countries, a 22% efficacy over typical CAP was reported (95% CI, 7.7–34.2).33
In Brazil, a country with low incidence of serotype 19A since the introduction of systematic vaccination with PCV10 there has been a 15% decrease in mortality from pneumonia in children under 24 months.34
23-valent pneumococcal polysaccharide vaccineThe 23-valent pneumococcal polysaccharide vaccine is still recommended in children over 2 years of age at risk for infection, although it probably has little impact on the prevention of CAP.
Vaccination against H. influenzae type bSince the introduction of the vaccine against Hib in the late 90s, there has been a drastic decrease of CAP caused by this microorganism. In some reports, a reduction of up to 30% of NACs has radiologically confirmed.35,36
Since non-typifiable H. influenzae is a very infrequent cause of CAP in previously healthy children, the PCV10 vaccine (due to its non-typifiable Hi component) probably has little impact.
Flu vaccineThe flu virus is a cause per se of CAP in the epidemic season. Also, in the cases of bacterial CAP co-infection with this virus is associated with a higher incidence of complicated forms,37 especially in cases where S. aureus is isolated, or no micro-organism is isolated.
According to current guidelines, the flu vaccine is recommended, for patients over 6 months of age with risk factors of complications, or for those living in the same household.38 Currently, the trivalent inactivated vaccine, which can be administered intramuscularly, is usually used in children. Some countries, such as the USA and the United Kingdom, have introduced the intranasal live attenuated influenza vaccine for children over 2 years of age with no history of bronchialhyperresponsiveness or asthma. This formulation will probably be available in Spain in the 2015–2016 vaccination campaign.
FundingThe authors declare they have not received any type of funding for this study.
Conflict of interestsConflict of interests of the authors as regards the document (in the last 5 years):
- –
DMP has collaborated in teaching activities funded by GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD, as a researcher in a clinical study conducted by Novartis and as a consultant on the Advisory Board of Astra-Zeneca and Pfizer.
- –
AAM presents no conflict of interests.
- –
ATG has collaborated in research activities funded by Pfizer.
- –
AEM has collaborated in teaching activities funded by Novartis, as a researcher in a multicentre study sponsored by GlaxoSmithKline and as a consultant in an Advisory Board of Gilead.
- –
JFM has collaborated in teaching activities funded by Gilead and Abbvie.
- –
JGG has collaborated in teaching activities funded by Pfizer and Sanofi Pasteur MSD.
- –
AMG has participated as a consultant in the Advisory Board of Abbvie and Gilead, has received institutional investigation grants from Abbvie and grants to attend congresses organised by Abbvie, Actelion, Ferrer, GlaxoSmithKline and Novartis.
- –
CRGL has collaborated in teaching activities funded by GlaxoSmithKline, Novartis, Pfizer and Sanofi Pasteur MSD, as a researcher in clinical trials conducted by GlaxoSmithKline and as a consultant on the Advisory Board of Astra-Zeneca, Novartis, GlaxoSmithKline and Pfizer.
- –
JRC has collaborated in teaching activities funded by GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD and as a researcher in clinical trials conducted by GlaxoSmithKline and Pfizer.
- –
JSL has collaborated as a researcher in clinical trials conducted by GlaxoSmithKline and Roche.
The affiliation of the authors is as follows:
| Respiratory Infections Work Group. Spanish Society for Paediatric Infectious Diseases (SEIP in Spanish): |
| D Moreno-Pérez, J.J. García García, C Rodrigo Gonzalo de Lliria, J Ruiz Contreras and J Saavedra Lozano |
| Spanish Society of Paediatric Pneumology (SENP in Spanish): |
| A Andrés Martín, A Escribano Montaner, J Figuerola Mulet, A Moreno-Galdó |
| Advisory Committee of the Paediatric Spanish Association (CAV-AEP in Spanish): |
| D Moreno-Pérez, J Ruiz Contreras |
Please cite this article as: Moreno-Pérez D, Martín AA, García AT, Montaner AE, Mulet JF, García JJG, et al. Neumonía adquirida en la comunidad: tratamiento ambulatorio y prevención. An Pediatr (Barc). 2015;83:439.e1–439.e7.