The gold standard for the diagnosis of pneumonia secondary to Mycoplasma pneumoniae is the serial measurement of IgM, since an isolated test for IgM has a poor sensitivity of 31.8%. A pneumonia due to M. pneumoniae could be of clinically different origins; thus, it is possible to perform a clinical score for its early diagnosis.
ObjectiveTo develop a clinical score in order to rule out a pneumoniae secondary to M. pneumoniae.
MethodologyA total of 302 patients from 0 to 18 years old, with a diagnosis of pneumonia were evaluated and divided into two groups: Mycoplasma positive and Mycoplasma negative. Using different variables in the medical records a clinical score was calculated.
ResultsOf the 302 cases studied, 34 were classified as Mycoplasma positive and 268 as Mycoplasma negative. The variables relevant to the calculation of the score were age, days with fever, and days with cough, thus providing the CAF (Cough, Age, Fever) score. Ranges were assigned for each variable and points were given for each range. A value greater than or equal to 5 meant a positive score. The CAF score was applied to the 302 cases, resulting in 164 cases of Mycoplasma positive and 138 cases of Mycoplasma negative. The CAF score had a sensitivity of 85% and specificity of 49%.
ConclusionThe CAF score had better sensitivity than other clinical diagnostic tools. With a negative predictive value of 96% it is possible to rule out a pneumonia secondary to M. pneumoniae. The study requires a prospective study to verify the usefulness of our score.
La prueba de oro para el diagnóstico de la neumonía secundaria a Mycoplama pneumoniae es la detección de IgM en pruebas seriadas, ya que una prueba aislada para IgM tiene una sensibilidad del 31,8%. Al existir un cuadro clínico diferenciable de la neumonía por M. pneumoniae de otras etiologías es posible realizar un score clínico para su diagnóstico temprano.
ObjetivoElaboración de un score clínico para el descarte de neumonía secundaria a M. pneumoniae.
MetodologíaSe evaluaron 302 expedientes; población de 0 a 18 años con diagnóstico de neumonía. Se obtuvieron 2 grupos: Mycoplasma positivo y Mycoplasma negativo, y utilizando distintas variables en la historia clínica se elaboró un score clínico.
ResultadosTreinta y cuatro casos se clasificaron en Mycoplasma positivo y 268 en Mycoplasma negativo. Las variables relevantes para la elaboración del score fueron edad, días con tos y días con fiebre, con lo que se conformó el score tos, edad, fiebre (TEF). Se asignaron rangos para cada variable y puntos para cada rango. Un valor de igual o mayor a 5 equivale a un score positivo. Se aplicó el score TEF a los 302 casos resultando ahora en 164 casos Mycoplasma positivo y 138 Mycoplasma negativo. Este score resultó en una sensibilidad del 85% y especificidad del 49%.
ConclusiónEl score TEF tuvo mejor sensibilidad que otras herramientas diagnósticas clínicas. Con un valur predictivo negativo del 96% es posible descartar anticipadamente una neumonía por M. pneumoniae. Se requiere realizar un estudio prospectivo para verificar la utilidad de nuestro score.
Lower respiratory tract infections are a major source of morbidity in paediatric patients.1Mycoplasma pneumoniae causes between 10% and 40% of the cases of community-acquired pneumonia (CAP).2 There is a higher prevalence in individuals aged 5–20 years. M. pneumoniae is a small, double-stranded DNA, aerobic, wall-deficient bacterium in the class Mollicutes that is found worldwide.3
Infection by M. pneumoniae is usually self-limiting and rarely fatal. Its clinical manifestations include a fever of 38.33–38.8°C with insidious onset, headache, general malaise, and cough, usually unproductive, possibly accompanied by parasternal chest pain. Physical examination commonly reveals an erythematous pharynx without the adenopathy characteristic of pharyngitis caused by group A streptococcus, and it is possible that no abnormalities are found on auscultation or percussion. Pleural effusion is found in 5–20% of the patients. However, it is important to consider that physical chest examination and chest radiography findings are not always in agreement. The most common extrapulmonary manifestations include bullous myringitis, Steven-Johnson syndrome, Raynoud's phenomenon, defects in cardiac conduction, and neurological manifestations such as encephalitis, meningitis, meningoencephalitis, transversal myelitis, and Guillain-Barré syndrome. The pathogen is transmitted via the oral route and has an incubation period of 2–3 weeks.4
The gold standard for diagnosing M. pneumoniae is detection of IgM antibodies in paired serum samples 2–3 weeks apart.5 It is common practise to perform a single test to detect IgM antibodies, but a single assay has a sensitivity of about 31.8%.5 Cold agglutinins serve as another diagnostic marker for M. pneumoniae. However, in children younger than 12 years old agglutinin serology is nonspecific and insensitive and thus is not recommended.6 There is also evidence that the clinical presentation of CAP due to M. pneumoniae is different from that of CAP for other aetiologies.7
Following the recommendations of the Guías del Centro Nacional de Excelencia Tecnológica en Salud (Guidelines of the National Centre for Technological Excellence in Health, CENETEC), management of CAP caused by M. pneumoniae in children older than five years is based on the use of macrolides to cover both typical and atypical agents.8 This indiscriminate use of macrolides has led to increased macrolide resistance in Streptococcus pneumoniae.9
ObjectiveSince there is no effective and quick method to diagnose M. pneumoniae and antibiotics must be used responsibly, this study aimed to develop a clinical score with which to rule out M. pneumoniae as the causative agent of CAP in the paediatric population.
MethodologyWe conducted a cross-sectional, observational, analytic study. We analysed the records of patients younger than 18 years diagnosed with CAP admitted to the Hospital San José-TEC of Monterrey between January 2010 and June 2012. We excluded patients who had been admitted previously to a different medical centre for treatment of their pneumonia. We entered the data for epidemiological, clinical, and paraclinical variables (Table 1) for each patient in a database using Microsoft Excel. Before analysing the medical records, the patients were divided into 4 groups according to the aetiology of their pneumonia as determined by various diagnostic tests: M. pneumoniae, bacterial, viral, and without aetiological diagnosis. We separated the cases without aetiological diagnosis into those in which testing was done and yielded negative results for viral or bacterial agents or for Mycoplasma (132 cases) and those that were diagnosed and treated empirically in which no testing was done (170 cases) (Table 2).
Clinical and paraclinical variables analysed.
| Analysed variables |
| Personal disease history |
| Previous symptoms |
| Previous treatment |
| Vital signs on admission |
| Physical examination |
| Laboratory and diagnostic tests |
| Chest radiograph |
| Treatment plan |
| Response to treatment |
| Hospital stay in days |
| Admission and days spent in paediatric intensive care unit and whether mechanical ventilation was required in this time |
Classification of pneumonia by aetiology as determined by diagnostic tests.
| Aetiology | Diagnostic test |
| Mycoplasma pneumoniae | Presence of serum IgM antibodies detected by immunoassay or cold agglutinin test |
| Bacterial | Positive culture of bronchoalveolar lavage, pleural fluid, or blood |
| Viral | Positive PCR or viral panel |
| Without aetiological diagnosis | An aetiology could not be established |
We analysed the variable data with the software STATA 11. We calculate the percentange, means, contingency tables, and the correlation of the variables under study. We performed a univariate analysis to find the odds ratio for each predictive factor (confidence intervals and P values). Then, based on the statistical results and on the work previously done by Fischer et al.,7 we developed a clinical scoring system to rule out M. pneumoniae as the cause of CAP in paediatric patients. We applied this scoring system tool to the sample of patients and calculated the sensitivity, specificity, negative predictive value, and positive predictive value of our score.
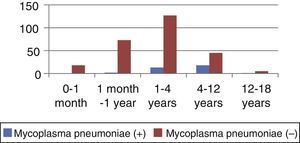
ResultsWe analysed the medical records of 302 patients diagnosed with CAP. The mean age was 2.92±3.11 years. The months with the highest incidence of CAP cases were December (19%), January (16%), and February and March, which had equal rates (14%). Out of all cases, 34/302 (11.26%) were classified as Mycoplasma-positive, and 268/302 (88.74%) as Mycoplasma-negative (Table 3). There were statistically significant differences in mean age (P<.001), the mean age being 5.52±3.33 years in Mycoplasma-positive patients and 2.59±2.92 years in Mycoplasma-negative patients. There was no difference in sex distribution between groups (P=.260). There were also significant differences in height and weight; however, these variables were directly correlated to age. The highest frequency of Mycoplasma positive cases was seen in children 1–4 years of age ([13/34], 9.29%). This difference was statistically significant (P<.001) (Table 4 and Fig. 1). We analysed the variables listed in Table 1 in the patients under study. We assessed several clinical and laboratory features to select which could be used as predictors. The features that showed the highest odds ratios were patient age, duration of fever in days, neutrophil count, C reactive protein (CPR) levels, and duration of cough in days (Table 5). Cough and fever were relevant symptoms. The presence of cough or fever alone was not significant (P=.168 and P=.54 respectively), but the duration in days of fever and cough prior to admission was statistically significant (P=.045 and P=.001 respectively). This is shown in Table 6. The other factors analysed showed no statistical significance.
Univariate analysis of aetiologies by age group: Mycoplasma positive and Mycoplasma negative.
| Age group | Mycoplasma pneumoniae (+) | Mycoplasma pneumoniae (−) | Total | Standard error | P value | 95% CI | ||
| Frequency | (%) | Frequency | (%) | |||||
| 0–1 month | 0 | – | 18 | 100 | 18 | |||
| >1 month–1 year | 2 | 2.6 | 73 | 97.33 | 75 | 0.07 | .303 | (95% CI: −0.2229637–0.0703541) |
| >1–4 years | 12 | 9.29 | 127 | 90.71 | 140 | 0.03 | <.001 | (95% CI: 0.0489987–0.1645799) |
| >4–12 years | 18 | 28.57 | 45 | 71.43 | 63 | 0.02 | .241 | (95% CI: −0.0200362–0.0782808) |
| >12–18 years | 1 | 16.67 | 5 | 83.33 | 6 | 0.11 | .740 | (95% CI: −0.0200362–0.0782809) |
| Total | 34 | 11.26 | 268 | 88.74 | 302 | |||
Logistic regression univariate analysis. The Mycoplasma (−) group includes cases without aetiological diagnosis, but only those with negative test results for Mycoplasma and other bacteria and viruses. Cases in which no testing had been done were not included in the analysis.
Univariate analysis of the clinical and laboratory variables in children with pneumonia caused by Mycoplasma compared with children with pneumonia of other aetiologies.
| Variable | Frequency | Odds ratio | Standard error | 95% confidence interval | P value |
| Age (years) | 132 | 1.57 | 0.14 | 1.32–1.87 | P<.001 |
| Fever (days) | 132 | 1.16 | 0.07 | 1.03–1.31 | P=.012 |
| Cough (days) | 132 | 1.06 | 0.03 | 0.99–1.13 | P=.055 |
| C reactive protein (g/dL) | 97 | 1.07 | 0.04 | 0.99–1.15 | P=.052 |
| Leukocytes (count×103/μL) | 130 | 1.04 | 0.04 | 0.97–1.11 | P=.283 |
| Neutrophils (count×103/μL) | 130 | 1.12 | 0.05 | 1.03–1.22 | P=.007 |
Univariate logistic regression analysis. The group not diagnosed with Mycoplasma only includes patients with negative test results for Mycoplasma and other bacteria and viruses.
Univariate analysis of the clinical characteristics of children with pneumonia caused by Mycoplasma compared with children with pneumonia of other aetiologies.
| Clinical characteristic | Mycoplasma pneumoniae (+) | Mycoplasma pneumoniae (−) | 95% CI | |
| Frequency | Frequency | P value | ||
| Patients with fever | 31 (91.18%) | 70 (71.43%) | P=.028 | (1.16832–14.62309) |
| Fever duration in days (±SD) | 6.19±10.68 | 2.37±3.12 | P=.012 | (1.033741–1.305508) |
| Patients with cough | 33 (97.05%) | 98 (90.82%) | P=.262 | (0.4069147–27.36715) |
| Duration of cough in days (±SD) | 8.07±10.68 | 4.90±5.35 | P=.055 | (0.9987652–1.117645) |
Univariate logistic regression analysis. The group not diagnosed with Mycoplasma only includes patients with negative test results for Mycoplasma and other bacteria and viruses.
The analysis we performed allowed us to develop a score using 3 clinical features of the patient. We called it CAF score as a mnemonic for the 3 parameters: cough, age, and fever. Cough and fever refer to the duration in days of these symptoms prior to admission. Table 7 shows the ranges for these parameters and the points assigned to each of these ranges to calculate the score.
Cough, age, and fever (CAF) score for ruling out pneumonia caused by Mycoplasma pneumoniae. Point assignment for each parameter.
| Age (years) | Points | Fever duration (days) | Points | Cough duration (days) | Points |
| <0.5 | 0 | <1 | 0 | <1 | 0 |
| 0.5–2 | 1 | 1–3 | 1 | 1–3 | 1 |
| >2–4 | 2 | >3–5 | 2 | >3–5 | 2 |
| >4–7 | 3 | >5–7 | 3 | >5–7 | 3 |
| >7–10 | 4 | >7–14 | 4 | >7–14 | 4 |
| >10 | 5 | >14 | 5 | >14 | 5 |
The final score is calculated by adding the points of each of the 3 items. A score of 5 or greater is considered a positive result.
After calculating the CAF scores in our database, we created a contingency table (Table 8), and the CAF score showed a sensitivity of 85%, a specificity of 49%, a negative predictive value of 96%, and a positive predictive value of 17%. Thus, this clinical tool could be used in cases of CAP to rule out pneumonia caused by M. pneumoniae.
DiscussionThe purpose of the study was to develop a clinical prediction rule that would allow for the differentiation of an atypical pneumonia secondary to M. pneumoniae, given the high prevalence of pneumonia caused by atypical organisms. For instance, Korppi et al. reported that infection by M. pneumoniae was detected in more than 30% of the paediatric patients and in more than 50% of the patients aged 5 years and older that had CAP, making it the most frequently detected pathogen.10
Although we analysed several variables to develop the score, such as physical examination findings, vital signs on admission, or chest radiography findings, the ones found to be relevant were age of the patient and duration of fever in days, which was consistent with the variables in the clinical score and decision tree published by Fischer et al.7 Our analysis also showed that duration of cough in days was another variable that should be included in the criteria.
When applied to the population under study, the CAF score had a sensitivity of 85%, a specificity of 49%, a negative predictive value of 96%, and a positive predictive value of 17%. Given these values we consider that the CAF score could be used as a clinical diagnostic tool to rule out pneumonia caused by M. pneumoniae, as opposed to the decision rule presented in the study by Fischer et al.,7 which was used to identify the risk of pneumonia due to M. pneumoniae.
The aim of our study was to provide a clinical tool to aid in the empirical prescription of an appropriate antibiotic in health facilities that do not perform laboratory tests for the timely identification of this pathogen, and thus contribute to the decline of antibiotic resistance in S. pneumoniae, the most common pathogen in CAP.
With a negative predictive value of 96% and a sensitivity of 85%, the CAF score could be used any time it is necessary to determine whether the causative agent of pneumonia is M. pneumoniae or a different microorganism. Some of the limitations of the study are that further research is required for broad validation of the score and to assess the score's performance in clinical practise by means of sensitivity analysis.
ConclusionOur CAF score had higher sensitivity than other diagnostic tools reported in the literature,7 and it had a considerable negative predictive value. This suggests that the CAF score can be used to rule out pneumonia by M. pneumoniae early compared to the gold standard, as it takes 2–3 weeks to obtain the test results in our setting, and a single serology test has a very low sensitivity. Ruling out pneumonia by M. pneumoniae in a timely manner would allow the administration of a specific antibiotic, so that a macrolide would not need to be used to cover typical and atypical agents. This would help prevent the spread of macrolide resistance in S. pneumoniae observed in different studies, such as PROTEKT US.9 We believe that the CAF score can be used both in outpatient settings and in the emergency room in children with suspected pneumonia caused by M. pneumoniae. The correct use of the CAF test allows ruling out CAP due to M. pneumoniae based on clinical parameters in healthcare settings where laboratory testing for M. pneumoniae is unavailable or where testing is available but results are considerably delayed. However, we must note that the usefulness of our score needs to be confirmed by means of a prospective study.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Rodríguez de Ita J, Torres-Quintanilla A, Paláu-Dávila L, Silva-Gburek JC, Ortiz de Elguea-Lizarraga J, Chávez Caraza KL, et al. Score clínico para el descarte de neumonía por Mycoplasma pneumoniae. Anales de Pediatría. 2014;81:241–245.
Previous presentations: Oral presentation at the Semana de Investigación, Innovación y Calidad en Salud 2012; September 25–28, 2012, Monterrey, Nuevo León, Mexico. Congreso Nacional de Pediatría (National Congress of Paediatrics). Poster presentation at the Congreso Nacional de Pediatría CONAPEME 2013; Monterrey, Nuevo León, Mexico. Participation in the xix Simposio de Investigación en Pediatría “Dr. Joaquín Cravioto Muñoz” in the context of the Congreso Nacional de Pediatría 2013 with an oral presentation that was awarded 3rd place nationwide within its category.