The benefits of donor human milk compared with artificial formulas have been well demonstrated; nevertheless the impact on the clinical practice of opening a human milk bank within a neonatal unit has not yet been studied. The main aim of this study was to analyse the impact on the clinical practice of opening a human milk bank in a neonatal unit to provide donor human milk for preterm infants ≤32weeks of gestational age.
MethodsA before and after study of the opening of a human milk bank was performed. Preterm infants ≤32weeks of gestational age born in the Hospital 12 de Octubre from July to December 2005 and January to June 2008 (firsts 6 months after opening the human milk bank) were included.
ResultsAfter opening the human milk bank, enteral feedings began 31h earlier (p<0.001), 100ml/kg/day were achieved 59.5h before (p<0.001) and 150ml/kg/day 52h before (p=0.002). Enteral feedings were never started LM with artificial formula, the exposure to formula in the first 15 days of life was reduced from 50% to 16.6%, and its consumption during the first 28 days of life was significantly reduced. There was a higher consumption of own mother's milk during the hospital stay, and a higher rate of exclusive breastfeeding at hospital discharge (54% vs 40%).
ConclusionsThe availability of donor human milk has led to quicker progression with enteral feedings and earlier withdrawal of parenteral nutrition. It has reduced the exposure to artificial formulas, and has also increased the intake of own mother's milk during the hospital stay and the rate of exclusive breastfeeding at hospital discharge.
Los beneficios de la leche donada frente a la fórmula artificial están demostrados, sin embargo no se conoce la influencia de la apertura de un banco de leche en la práctica clínica habitual. El objetivo de este estudio fue medir el impacto en la práctica clínica de la disponibilidad de leche donada para la nutrición de los prematuros ≤32semanas de edad gestacional.
MétodosEstudio antes-después de la apertura de un banco de leche. Se incluyeron los ≤32semanas nacidos en el Hospital 12 de Octubre de julio-diciembre de 2005 y de enero-junio de 2008 (6 primeros meses tras la apertura del banco de leche).
ResultadosLa apertura del banco de leche permitió empezar 31h antes (p<0,001) la alimentación enteral, se alcanzaron 59,5h antes los 100ml/kg/día (p<0,001) y 52h antes los 150ml/kg/día (p=0,002), permitiendo retirar 72h antes la nutrición parenteral. En ningún prematuro se inició la alimentación enteral con fórmula artificial, la exposición a la misma en los primeros 15días de vida bajó del 50 al 16,6% y su consumo durante los primeros 28días fue significativamente menor. La cantidad consumida de leche de la propia madre fue mayor, al igual que la tasa de lactancia materna exclusiva al alta (54 vs 40%).
ConclusionesDisponer de leche donada ha permitido avanzar más rápidamente con la nutrición enteral y retirar antes la nutrición parenteral. La exposición a fórmula artificial ha sido menor y mayor el consumo de leche de madre propia y la lactancia materna al alta.
Nutrition is one of the main pillars of care for hospitalised infants, especially in those born preterm. As stated by the WHO, the best food for the infant is the mother's own milk (MM), whose benefits over artificial formula have been established both in healthy and ill children. If MM is not available, the next best choice recommended for very-low-weight preterm infants is pasteurised human donor milk (DM) from selected healthy donors,1 if available.
Enteral feeding with artificial formula milk has been associated with an increased risk for necrotising enterocolitis (NEC) in high-risk infants, especially very low weight preterm infants, compared to feeding with MM.2–8. Already in 1990 Lucas established that NEC was 6–10 times more prevalent in patients fed exclusively with artificial formula, compared to those who were fed exclusively with MM, and 3 times more prevalent in those exclusively formula-fed compared to those fed with a combination of formula and breastmilk.9 There is evidence, although not as strong, that MM protects against nosocomial infection, reducing the number of infectious episodes.4 Other long-term benefits have been observed, such as improved psychomotor development outcomes,8,10,11 and a decrease in cardiovascular disease risk factors.8,12,13
However, so far, few studies have been published that analyse other potential benefits of the availability of DM in neonatal units. As a milk bank was about to open in our institution, we thought of the need of conducting a study on the immediate clinical impact of the availability of DM, especially in relation to the duration of parenteral feeding, the time elapsed to removal of central lines, and the impact on breastfeeding. Since the beneficial effects of MM are well known, it seemed unethical to do a randomised trial and not administer DM to all possible recipients once it became available.
The milk bank in our centre opened in December 2007. The aim of this study was to assess the clinical impact of having pasteurised DM available to feed preterm infants born at gestational ages ≤32 weeks.
MethodsDesignWe performed a prospective quasi-experimental pre- and post-intervention study. The intervention consisted of the availability of DM following the opening of a milk bank in the neonatology unit that could meet the full requirements of the population under study.
Parental consentPrior to administering DM to patients, one of the parents or legal guardians signed an informed consent form after receiving verbal and written information. DM was always administered by medical prescription, for the duration that the physician in charge of the patient considered necessary. DM was given only when MM was not available.
Study groupsGroup I: born from July to December 2005; Group II: born from January to June 2008 (the first 6 months of operation of the milk bank).
Population under studyWe included all preterm infants born at gestational ages ≤32 weeks in our maternity unit, with the exception of those who had chromosopathies, genetic diseases, major malformations, and those who died within 7 days from birth.
Study variablesPrimary variables: hours of life at which enteral feeding starts; hours of life at which the infant reaches 24ml/kg/day, 100ml/kg/day, and 150ml/kg/day of enteral nutrition; hours receiving parenteral nutrition in the first 28 days of life; hours with central lines in the first 28 days of life; volume of enteral nutrition administered. Secondary variables: number of confirmed sepsis episodes; days of antibiotic or antifungal treatment in the first 28 days of life; number of NEC episodes (Bell's stage II or higher) and surgical treatment of NEC; bronchopulmonary dysplasia at 36 weeks of corrected age; patent ductus arteriosus; weekly and discharge weight z-score.
We retrieved the data from the clinical histories of patients at the time of hospital discharge.
Result analysisWe described quantitative variables by means of the mean and median, and dispersion parameters (standard deviation or maximum and minimum). We expressed qualitative values in percentages. The Kolmogorov–Smirnov test was used to test the normality of the distributions. We compared quantitative variables that followed normal distributions with Student's t-test, and the non-parametric Mann–Whitney test for the remaining quantitative variables. Qualitative variables were analysed with the chi-squared test. We used Fisher's test whenever the proportion of a result was <5%. We set our alpha level at 0.05.
The study was approved by the centre's Clinical Research Ethics Committee.
ResultsDuring the first period of the study, 54 children were born that met the inclusion criteria, and 6 were excluded after dying in the first week of life. Thus, Group I consisted of 48 patients, none of whom died during their hospital stay. In the second period, 52 patients were born that met the inclusion criteria, 4 of whom died in the first week. Thus, Group II consisted of 48 infants, 2 of whom died during their hospital stay after the first week of life. The demographic characteristics of both groups are summarised in Table 1.
Demographic characteristics of the 2 groups under study.
| Group I (n=48) | Group II (n=48) | p | |
| Gestational age (weeks), mean (SD) | 29.2 (1.7) | 28.8 (1.9) | 0.29 |
| Birth weight (g), mean (SD) | 1.220 (317) | 1.187 (318) | 0.63 |
| Sex, male, N (%) | 23 (48) | 27 (56) | 0.41 |
| Twins, N (%) | 21 (44) | 15 (31) | 0.21 |
| Mother's age (year), mean (SD) | 31.9 (5.5) | 30.9 (6.1) | 0.37 |
| Caesarean, N (%) | 40 (83) | 34 (71) | 0.15 |
| Hospital stay in days, mean (SD) | 55.9 (24) | 56.9 (2.7) | 0.85 |
| Corrected age at discharge, mean (SD) | 36.9 (2.6) | 36.9 (2.7) | 1 |
Enteral feeding was started at a mean age of 21h in Group II (range: 3–148h), and at 52h in Group I (range: 15–356h). The difference was significant (p<0.001). Group II also reached 24ml/kg/day of enteral nutrition earlier (Group II median: 61h, range: 26–278h; Group I median: 120h, range: 48–480h; p<0.001), as well as 100ml/kg/day (Group II median: 156.5h, range: 73 to 556h; Group I median: 216h, range: 120 to 1200h; p<0.001), and 150ml/kg/day (Group II median: 248h, with range 139 to 855h; Group I median: 300h, range: 216–1296h; p=0.002).
In Group II, none of the patients were fed preterm formula (PF) enterally. Enteral feeding was initiated with MM in 42% of these patients (20/24, with DM in another 42% (20/48), and with a combination of both types of milk in the remaining 16% (8/48). In Group I, enteral nutrition started with MM in 64% (31/48) of patients, with DM in 21% (10/48), and with a combination of both in the remaining 15% (7/48). We defined “initiation of feeding” as the 24-h period following the first enteral feed.
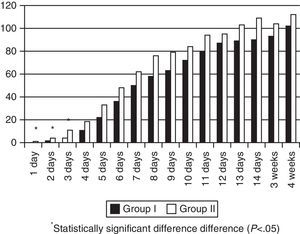
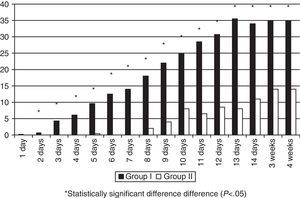
MM intake was greater in Group II in the first days of life, but there were no significant differences during the rest of the hospitalisation (Fig. 1). The patients exposed to artificial formula in the first 14 days of life comprised 16.6% (8/48) of patients in Group II and 50% (24/48) of patients in Group I (p=0.0005). None of the Group II newborns consumed PF in the first four days of life, and the maximum intake in the first week of life was 0.3ml/kg/day. Artificial formula intake was significantly lower in Group II for the first 4 weeks of life starting at 24h from birth (Fig. 2).
Group II patients required parenteral nutrition for a mean of 193±157h, and Group I patients for 265±221h (p=0.071). Central lines were kept for 172±120h in Group II and for 200±180h in Group I (p=0.38).
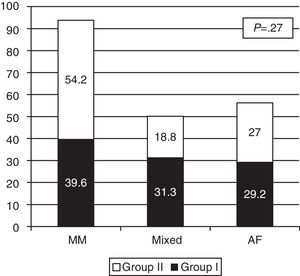
The proportions of exclusive or mixed breastfeeding at discharge in both groups are shown in Fig. 3. Although the differences are not statistically significant, there was a higher rate of exclusive breastfeeding at discharge in Group II: 54% (26/48) versus 39% (19/48) in Group I.
When it came to morbidity during hospitalisation, there were no significant differences in the number of sepsis episodes per patient confirmed by blood culture (0.29 in Group II vs 0.31 in Group I; p=0.7), or in the proportion of patients with infections (27% in Group II vs 31% in Group I; p=0.65). We also found no significant difference in the number of days of antibiotic treatment (Group II median: 5 days; Group I median: 7.5 days; p=0.079). However, Group II received fewer antifungal drugs (5.2±7.6 days vs 2.4±5; p=0.04).
The incidence of NEC was lower in Group II (2%) than in Group I (8.3%; p=0.3) but the difference was not statistically significant. The difference for combined NEC and isolated intestinal perforation was statistically significant (Group II: 2%; Group I: 12.5%; p=0.049). We did not find differences in the frequency of patent ductus arteriosus treated with medication or surgery, nor in the prevalence of bronchopulmonary dysplasia at discharge (Table 2).
Morbidity during hospitalisation in the two study periods.
| Variable | Group I | Group II | p |
| PDA+indomethacin | 20/48 (41.7%) | 16/48 (33.3%) | 0.4 |
| PDA+surgery | 5/48 (10.4%) | 2/48 (4.2%) | 0.44 |
| NEC | 4/48 (8.3%) | 1/48 (2%) | 0.3 |
| NEC+perforation | 6/48 (12.5%) | 1/48 (2%) | 0.049 |
| BPD 36wk | 6/48 (12.5%) | 8/46 (17.4%)a | 0.5 |
BPD 36wk, bronchopulmonary dysplasia at 36 weeks of corrected age; NEC, necrotising enterocolitis; PDA, patent ductus arteriosus.
Data are expressed as absolute frequencies (percentages).
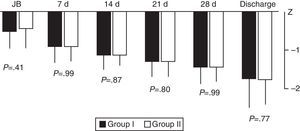
We did not find significant differences between the groups in weight gain during hospitalisation based on the weekly calculation of the weight z-score from birth to discharge (Fig. 4).
We also did not observe significant differences in the length of hospital stay (Group II median: 57 days, range: 16–144; Group I median: 51 days, range: 25 to 165; p=0.8). The mean corrected age at discharge was the same in both groups: 36.9 weeks.
DiscussionOpening the milk bank in the neonatal unit allowed earlier initiation of enteral feeding in preterm newborns in a unit where efforts were being made before to start feeding earlier with MM. Since it became unnecessary to wait for MM to be available, enteral feeding was started 31h earlier if we consider the median, and 36h earlier if we consider the mean. This is one of the advantages of having human milk available from birth.14 As digestive tolerance is better with human milk than with artificial formula and there was no need to wait for MM availability, enteral feeds could be advanced faster so that a volume of 100ml/kg/day was reached much earlier (59.5h before if we consider the median, and 105h if we consider the mean), as was 150ml/kg/day (52h earlier if we consider the median, and 101h earlier if we consider the mean). These findings are consistent with the results of a previous study that found that patients fed mostly with human milk advanced to 150ml/kg/day 5 days earlier than those who consumed a low proportion of human milk.15
By reaching full enteral feeding earlier, parenteral nutrition can be discontinued earlier, and the central lines can be removed earlier as well. Our study did not find significant differences in this regard, but the trend is clear and would probably reach statistical significance in a larger sample.
There is strong evidence that feeding this population with artificial formula increases their risk of NEC, and several studies have found an association with other risks in the short and the long term.2–8 The literature describes periods in which exposure is particularly critical.16 The first one is the initiation of feeding, when colostrum plays its role. Although part of the beneficial effects of colostrum is lost when feeding is initiating with pasteurised mature milk, at least it can prevent exposure to artificial formula. The availability of DM did not result in delayed milk expression by the mothers; in fact, the amount of MM consumed in the first 3 days of life was greater than before, and the difference was statistically significant.
The restriction of the early use of artificial formulas is important in this population. A dose–response relationship has been found between the amount of MM consumed in the first 4 weeks of life and the decrease in morbidities characteristic of preterm babies, such as digestive intolerance, nosocomial infection, NEC, retinopathy of prematurity, and bronchopulmonary dysplasia.14–17 Or from another perspective, the greater the exposure to artificial formulas, the greater the risk. Consumption of artificial formula in the first 4 weeks of life decreased significantly, and it was practically nonexistent in the first week (see Figs. 1 and 2). The increase in the consumption of MM that took place was not statistically significant, but sufficed to counter one of the arguments of milk bank opposers: the availability of DM does not necessarily result in decreased efforts to promote breastfeeding in the units. A 54% rate of exclusive breastfeeding combined with a 19% rate of mixed breastfeeding at discharge can be considered a success. A multicentre study performed in 8 regions of Europe found rates of exclusive breastfeeding at discharge that ranged from 29% in Trento (Italy) and 9% in Poland.18 Another study19 analysed 12 level-3 neonatal units in Italy, and found an exclusive breastfeeding rate at discharge that ranged between 0 and 69%. Another study in Italy compared breastfeeding rates at discharge in units that had a milk bank and units that did not,20 and found that exclusive breastfeeding rates were higher in units with milk banks (29.6% vs 16%). And one study performed in 99 intensive care units in California21 found an average exclusive breastfeeding rate of 62.5% at discharge.
We did not find differences in the incidence of sepsis nor antibiotic treatment. The decrease observed in antifungal drug administration was probably due to changes in unit protocols due to the decreased frequency of invasive Candida infections in recent years.
Some authors found that infants fed with MM during hospitalisation were discharged earlier, even if they had smaller weight gains,22 possibly due to less severe morbidity during their stay. The literature has also reported lower weight gains in preterm babies fed with pasteurised DM compared to babies fed with their mother's own raw milk.23 Our study did not find differences between these groups.
The lower prevalence of enterocolitis or intestinal perforation found in the study may have been influenced by the fact that several cases in the first period were associated to rotavirus infection, which was not the case during the second period. The 12.5% rate is not habitual in the unit, and the usual NEC rate for the unit in this population ranges between 2% and 3%.
This study has several limitations. The first one is that the intervention was not randomised due to the reasons explained above. Another limitation is that we have compared 2 groups of patients in time periods that are relatively far apart, so we cannot rule out that the observed results are associated to other changes in the clinical practices of the unit. The reason for this gap is that the study was designed in 2005 with the expectation that funding would be available to open the milk bank in 2006. As the funding was delayed, the Group II sample was obtained 3 years later, covering the first six months of operation of the milk bank.
Despite its limitations, our study contributes additional data to the scarce information available on the clinical impact of the opening of a milk bank in a neonatal unit.24 In order to generalise these results, it would be good to obtain data from other banks and neonatal units. In any case, it seems clear that the availability of DM significantly reduces the number of newborns exposed to formula at a critical period of their development, and makes enteral feeding of these newborns possible at an earlier time.
Conflicts of interestThe authors have no conflicts of interest to declare.
Presented as: Presentation at the 1st International Congress of the European Milk Bank Association (EMBA), Lisbon (Portugal), October 5–6, 2012. One oral communication at the VI Congress of the Spanish Breastfeeding and 3rd Meeting of Human Milk Banks, Avila April 7–9, 2011. Two oral communications at the XXII Congress of Neonatal and Perinatal Medicine of the Spanish Society of Neonatology, Valencia, October 14–16, 2009.
Please cite this article as: Vázquez-Román S, Bustos-Lozano G, López-Maestro M, Rodríguez-López J, Orbea-Gallardo C, Samaniego-Fernández M, et al. Impacto en la práctica clínica de la apertura de un banco de leche en una unidad neonatal. An Pediatr (Barc). 2014;81:155–160.