Therapeutic hypothermia (TH) improves survival and neurological prognosis in hypoxic-ischemic encephalopathic (HIE) babies, being better the sooner TH is implemented. HIE babies are born more frequently in a non-cooling centre and need to be referred.
MethodsProspective-observational study (April 18 2018 – November 19 2019). Newborns (≥34 weeks of gestational age (GA) and >1800 g) with moderate/severe HIE on non-servocontrolled therapeutic hypothermia by the two neonatal transport teams in Catalonia.
Results51 newborns. The median stabilisation and transport time were 68 min (p25–75, 45–85 min) and 30 min (p25–75, 15–45 min), respectively. The mean age at arrival at the receiving unit was 4 h and 18 min (SD 96.6). The incubator was set off in 43 (84%), iced-packs 11 (21.5%) and both (11, 21.5%). Target temperature was reached in 19 (37.3%) babies. There were no differences in the overcooling in relation to the measures applied. The transport duration was not related with temperature stabilisation or target temperature reachiness.
ConclusionsRectal temperature monitorisation is compulsory for the stabilisation and the application of non-servocontrolled hypothermia during transport. There is still time for improving in the administration of this treatment during transport. Servo-controlled hypothermia would be a better alternative to improve the management of HIE babies.
La hipotermia terapéutica (HTT) es el único tratamiento que ha demostrado aumentar la posibilidad de supervivencia libre de secuelas en los recién nacidos (RNs) afectos de encefalopatía hipóxico-isquémica (EHI), recomendándose iniciarla lo antes posible. Lo más frecuente es que los pacientes tributarios de HTT no nazcan en los centros de referencia (CR), requiriendo ser transportados.
MétodosEstudio observacional descriptivo prospectivo de RNs con EHI moderada-grave trasladados en hipotermia terapéutica no servo-controlada por los dos equipos de transporte neonatal y pediátrico terrestres de Cataluña (abril 2018-noviembre 2019).
Resultados51 pacientes. Mediana de tiempo de estabilización 68 minutos (p25-75, 45 – 85 min), traslado 30 minutos (p25-75, 15 – 45 min). Media de edad a la llegada al CR 4 horas y 18 minutos (DE 96 min). Medidas terapéuticas adoptadas: apagar la incubadora 43 (84,3%), bolsas de hielo 11 (21,6%) y ambas 11 (21,5%) pacientes. Se consiguió la temperatura rectal (TR) diana en 19 (37,3%) pacientes. No hubo diferencias en el sobre-enfriamiento según las medidas usadas para la aplicación de la HTT no servo-controlada (HTTnc). La duración del traslado no se relacionó con diferencias en la estabilización de la temperatura ni en la consecución de la temperatura objetivo.
ConclusionesLa monitorización de la TR en el centro emisor es un pilar fundamental en la estabilización del paciente y la aplicación de la HTTnc. Existe una clara área de mejora en la eficacia de la HTTnc durante el transporte. La HTT servo-controlada sería una opción para poder ofrecer las mismas posibilidades terapéuticas a los RNs extramuros de los CR.
Hypoxic-ischaemic encephalopathy (HIE) is a heterogeneous illness characterised by early central nervous system dysfunction in neonates secondary to a hypoxic-ischaemic event and manifesting with changes in the level of consciousness accompanied by difficulty initiating or maintaining respiration and depressed tone and reflexes.1–4 There are 3 stages of HIE: primary energy failure, latency period (characterised by a transient recovery of aerobic metabolism) and secondary energy failure. The last stage typically starts about 6 hours after the event with the accumulation of excitatory neurotransmitters and damage secondary to oxidative stress and inflammation. The resulting brain damage leads to programmed cell death lasting hours or days, and its severity is strongly associated with the risk of adverse neurodevelopmental outcomes.5 Therapeutic hypothermia (TH), which is applied in the latency period,3,4,6 is the only treatment proven to increase the probability of survival free of sequelae in neonates with moderate-to-severe HIE,6–8 with treatment of a relatively low number of patients required to prevent one case of disability or death (number needed to treat [NNN] = 7).7,9 In Spain, this treatment has been recommended since 2011 and is delivered by referral hospitals (RHs).1,3
The incidence of moderate/severe HIE in Spain is of 0.77 cases per 1000 live births.2 It is estimated that 55 neonates develop moderate/severe HIE in Catalonia each year. Patients eligible for TH are not usually born in RHs, which makes optimal management and stabilization at the sending hospital (SH) and during neonatal transport (NT) to the RH where they will receive definitive care.4,10 Despite the increasing efficacy of TH in reducing morbidity, probably due to an increase in the number of patients with moderate HIE receiving this treatment in addition to early initiation of TH,11,12 HIE continues to cause substantial neonatal morbidity and mortality and is the leading cause of disability in terms of disability-adjusted life years worldwide.3,4 The factors that contribute to its efficacy are the time of initiation, achievement of the target temperature (Tt) and the duration of cooling.3,11,13,14 Cooling should be initiated as early as possible,4,15 even in neonates requiring transport to the RH, starting cooling at the sending hospital and continuing through transport. An essential aspect is the identification of patients eligible for TH and the control of potential associated health problems, thus establishing the so-called chain of neuroprotection as soon as possible and within the 6-hour period known as the golden window.16 The need to continue TH will be assessed on arrival to the RH, which will have more information available from the continuous observation of neurologic manifestations and diagnostic methods such as amplitude-integrated encephalography and transfontanellar ultrasound. Several studies have highlighted the difficulty of differentiating mild from moderate HIE in a single clinical assessment, as a case can be initially classified as mild but then progress with features compatible with moderate HIE.3,4,17–20 In addition, infants with mild HIE are also at risk of increased morbidity in the long term, and the applicability of TH to their management has yet to be determined.3,17–22
Paediatric and neonatal transport teams (PNTTs) must provide effective and safe transport to neonates with HIE allowing early initiation of TH, avoiding the risk of overcooling and other complications and guaranteeing the earliest possible arrival to the RH. The Medical Emergency System of Catalonia includes two paediatric ground transport units, both headquartered in Barcelona (Hospital Sant Joan de Déu and Hospital Vall d’Hebrón), and serving an area of 33 000 km2 with 70 000 births per year in the territories of Catalonia and Andorra. They carry out approximately 1400 transports per year (50% neonatal transports). The PNTT includes one emergency medical technician specialised in paediatric transport, one paediatric nurse and one paediatrician, all of them trained on neonatal care.
The aim of the study was to describe the management of non-servocontrolled therapeutic hypothermia (nscTH) in neonates with HIE eligible for this treatment and transported by the 2 PNTTs.
MethodsWe conducted a prospective observational descriptive study in patients that met the criteria for perinatal hypoxia-ischaemia with clinical manifestations compatible with moderate/severe HIE transported by the ground PNTTs between April 2018 and November 2019.
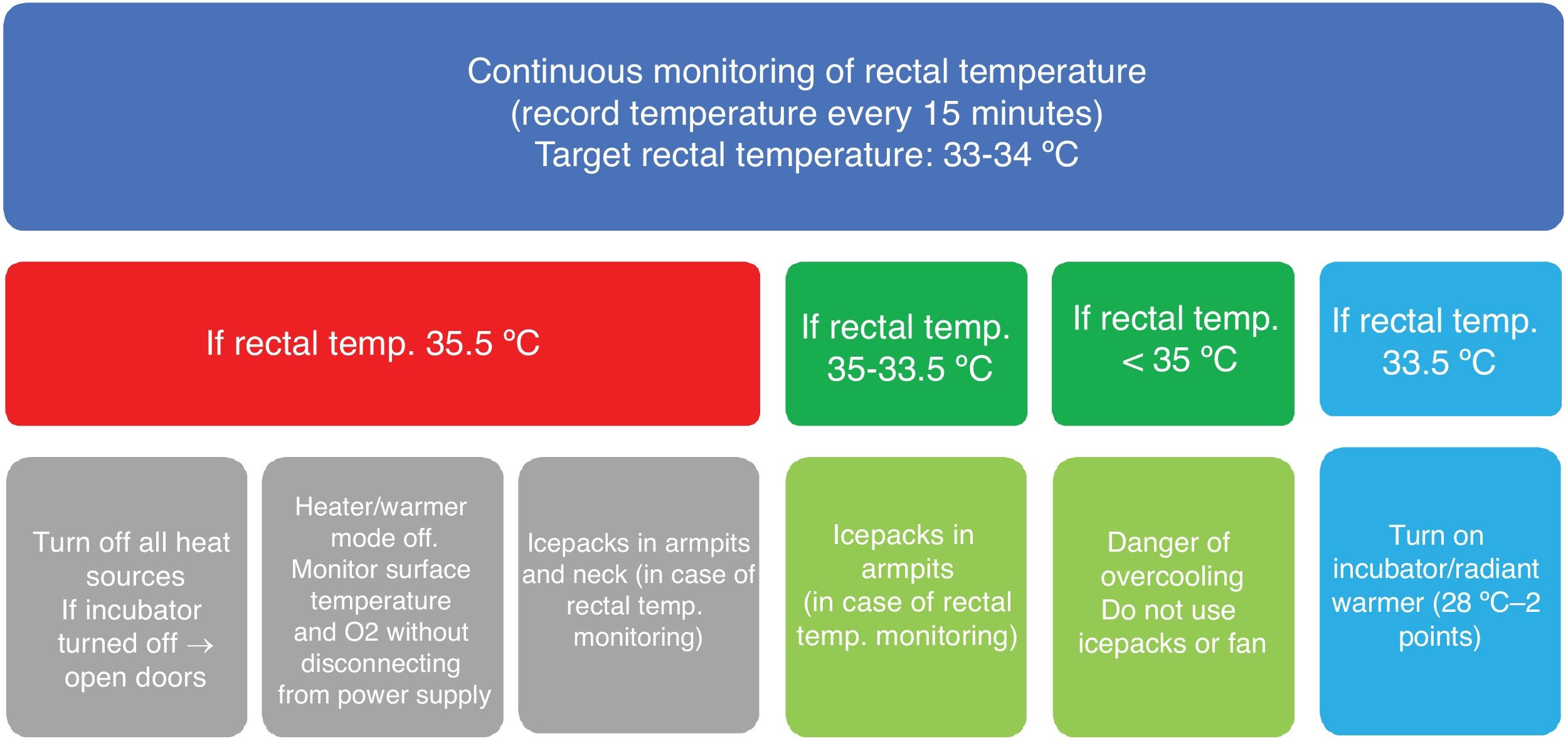
Before the start of the study, the protocol for use of nscTH was established by consensus (Fig. 1) and transport teams were trained on the use of the HIE assessment scale developed by Alfredo García-Álix.
The study was approved by the clinical research ethics committees of both hospitals (files PIC137-17 and ID-RT065). We obtained signed informed consent to include patient data from the legal guardians of the patients.
We included neonates born at 34 or more weeks of gestation with birth weights greater than 1800 eligible for TH. The diagnostic criteria for definition of perinatal hypoxia/ischaemia were: Apgar score of less than 5 at 5 minutes post birth, pH below 7 or base excess of less than −16 mmol/L in a sample of foetal blood, cord blood or peripheral blood obtained within 1 hour of birth, need for advanced life support (positive airway pressure for more than 5 minutes, intubation, cardiac massage, vasoactive drugs) or sudden unexpected postnatal collapse. The assessment of encephalopathy consisted of an exhaustive neurologic examination with evaluation of level of consciousness, muscle tone, motor reflexes, primitive reflexes, presence of abnormal movements (clonus, jerky movements, tremors, convulsions) and the pupillary reflex. We also collected data on perinatal clinical characteristics, treatment and axillary temperature (aT) and/or rectal temperature (rT) on arrival and departure from SH, during transport (at 15-minute intervals) and on arrival to the RH. Rectal temperature was monitored continuously with a Smiths Medical Level 1® probe. We classified rTs as overcooling (< 33 °C), target temperature (33-34 °C) and warm (> 34 °C). We defined stable rT during transport as the rT not moving between categories for its duration. We collected data on the time to stabilization (arrival to SH to departure from SH) and transport time (departure from SH to arrival to RH).
We excluded infants with neonatal encephalopathy unrelated to hypoxia/ischaemia at birth, major congenital malformations or suspected chromosomal abnormalities, congenital metabolic disorders, neuromuscular disorders (including spinal cord injuries), born before 34 weeks’ gestation and/or with a birth weight under 1800 g.
We conducted a descriptive analysis of the sample. We calculated the mean, standard deviation and standard error and recorded the minimum and maximum values for quantitative variables. For quantitative data with nonparametric distributions, we calculated the median and interquartile range (IQR). We compared proportions with the chi square test or Fisher exact test as applicable. In the case of quantitative data that was not normally distributed, we used the Kruskal-Wallis and Mann-Whitney U tests. We defined statistical significance as a p-value of less than 0.05 (two-tailed). The statistical analysis was performed with the software SPSS version 24.
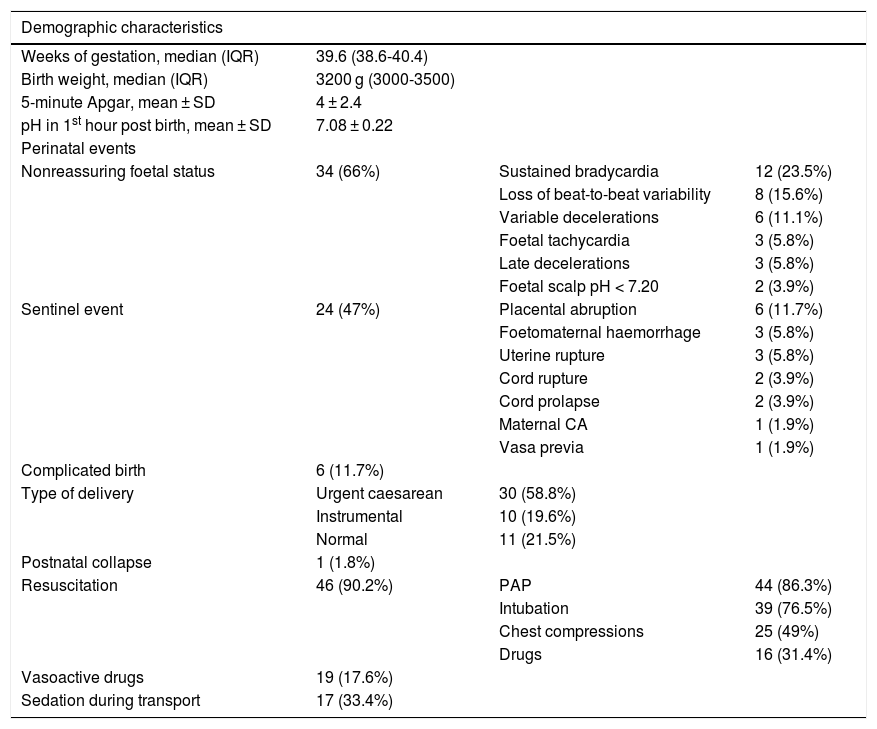
ResultsA total of 59 neonates met the criteria for HIE. We excluded 8 due to missing data (all needed mechanical ventilation and 6 vasoactive drugs). The sample included 51 patients whose demographic and clinical characteristics are summarised in Table 1. Five (9.8%) were delivered between 34 and 36 weeks’ gestation. Thirty-four (66%) had 5-minute Apgar scores under 5 points and 32 (62.7%) clinically significant acidosis in the first hour post birth. In 10 patients (19.6%), there was a history of complications during pregnancy, most frequently gestational diabetes (4 patients). The level of consciousness was normal in 12 patients (22.2%), with lethargy in 11 (21.6%), obtundation in 11 (21.6%), coma in 8 (14.8%) and stupor in 7 (13.7%), and was unknown in 2 (3.8%). Twenty-seven patients had hypotonia (52.9%) and 12 hypertonia (23.5%). An adequate neurological examination could not be performed in 11 patients (21.6%) because they required sedation or analgesia. Twelve patients (23.5%) had convulsions, which were clonic in 8 and subtle in 5.
Demographic and perinatal characteristics of transported neonates with moderate-to-severe HIE that underwent cooling.
| Demographic characteristics | |||
|---|---|---|---|
| Weeks of gestation, median (IQR) | 39.6 (38.6-40.4) | ||
| Birth weight, median (IQR) | 3200 g (3000-3500) | ||
| 5-minute Apgar, mean ± SD | 4 ± 2.4 | ||
| pH in 1st hour post birth, mean ± SD | 7.08 ± 0.22 | ||
| Perinatal events | |||
| Nonreassuring foetal status | 34 (66%) | Sustained bradycardia | 12 (23.5%) |
| Loss of beat-to-beat variability | 8 (15.6%) | ||
| Variable decelerations | 6 (11.1%) | ||
| Foetal tachycardia | 3 (5.8%) | ||
| Late decelerations | 3 (5.8%) | ||
| Foetal scalp pH < 7.20 | 2 (3.9%) | ||
| Sentinel event | 24 (47%) | Placental abruption | 6 (11.7%) |
| Foetomaternal haemorrhage | 3 (5.8%) | ||
| Uterine rupture | 3 (5.8%) | ||
| Cord rupture | 2 (3.9%) | ||
| Cord prolapse | 2 (3.9%) | ||
| Maternal CA | 1 (1.9%) | ||
| Vasa previa | 1 (1.9%) | ||
| Complicated birth | 6 (11.7%) | ||
| Type of delivery | Urgent caesarean | 30 (58.8%) | |
| Instrumental | 10 (19.6%) | ||
| Normal | 11 (21.5%) | ||
| Postnatal collapse | 1 (1.8%) | ||
| Resuscitation | 46 (90.2%) | PAP | 44 (86.3%) |
| Intubation | 39 (76.5%) | ||
| Chest compressions | 25 (49%) | ||
| Drugs | 16 (31.4%) | ||
| Vasoactive drugs | 19 (17.6%) | ||
| Sedation during transport | 17 (33.4%) |
CA, cardiac arrest; HIE, hypoxic-ischaemic encephalopathy; PAP, positive airway pressure; SD, standard deviation.
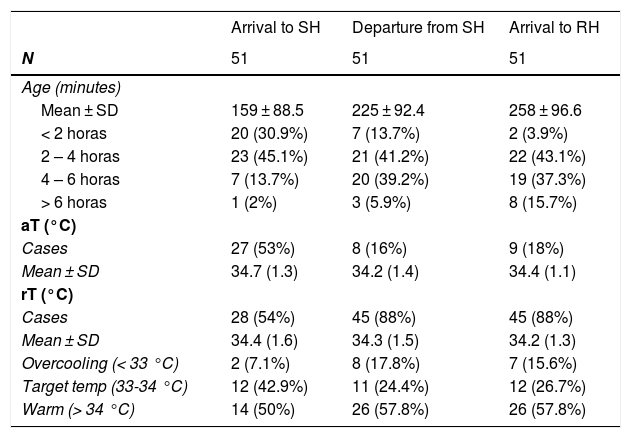
The median time to stabilization was 68 minutes (IQR, 45-85) and the median transport time 30 minutes (IQR, 15-45); with a transport time of less than 15 minutes in 12 patients (23.5%), 15 to 45 minutes in 26 (51%) and more than 45 minutes in 13 (25.5%). Table 2 presents the mean ages of the patients, with a mean age of 4 hours and 18 minutes at the time of arrival to the (SD, 96 minutes).
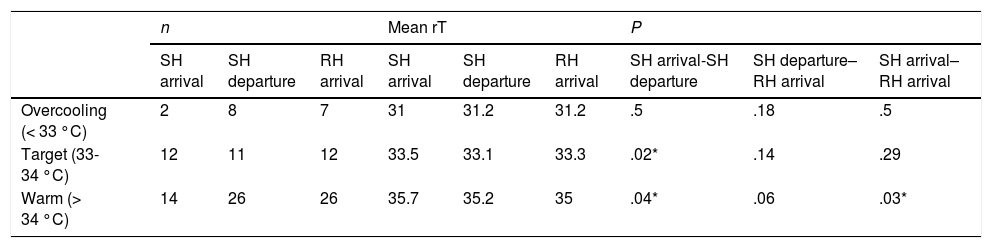
Temperature monitoring and category distribution during transport.
| Arrival to SH | Departure from SH | Arrival to RH | |
|---|---|---|---|
| N | 51 | 51 | 51 |
| Age (minutes) | |||
| Mean ± SD | 159 ± 88.5 | 225 ± 92.4 | 258 ± 96.6 |
| < 2 horas | 20 (30.9%) | 7 (13.7%) | 2 (3.9%) |
| 2 – 4 horas | 23 (45.1%) | 21 (41.2%) | 22 (43.1%) |
| 4 – 6 horas | 7 (13.7%) | 20 (39.2%) | 19 (37.3%) |
| > 6 horas | 1 (2%) | 3 (5.9%) | 8 (15.7%) |
| aT (°C) | |||
| Cases | 27 (53%) | 8 (16%) | 9 (18%) |
| Mean ± SD | 34.7 (1.3) | 34.2 (1.4) | 34.4 (1.1) |
| rT (°C) | |||
| Cases | 28 (54%) | 45 (88%) | 45 (88%) |
| Mean ± SD | 34.4 (1.6) | 34.3 (1.5) | 34.2 (1.3) |
| Overcooling (< 33 °C) | 2 (7.1%) | 8 (17.8%) | 7 (15.6%) |
| Target temp (33-34 °C) | 12 (42.9%) | 11 (24.4%) | 12 (26.7%) |
| Warm (> 34 °C) | 14 (50%) | 26 (57.8%) | 26 (57.8%) |
aT, axillary temperature; RH, receiving hospital; rT, rectal temperature; SD, standard deviation; SH, sending hospital; tT, target temperature.
Non-servocontrolled TH was delivered by turning off the incubator in 43 patients (84.3%), applying icepacks in 11 (21.6%) and both in 11 (21.6%). The target rT was achieved in 19 patients (37.5%), of who 12 (23.5%) reached the RH with temperatures in the target range. The median age on arrival to the RH was 70 min (IQR, 50–180).
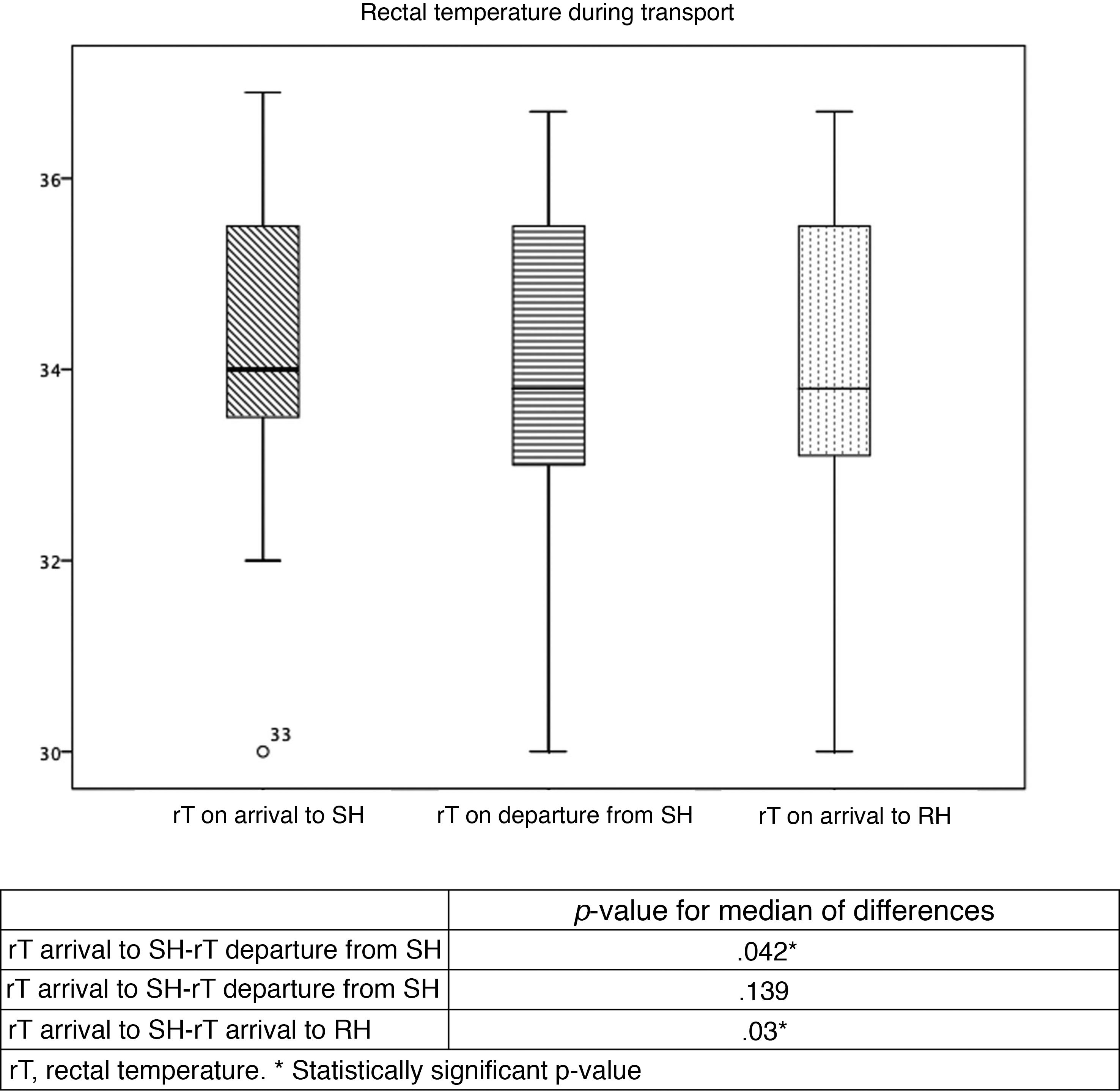
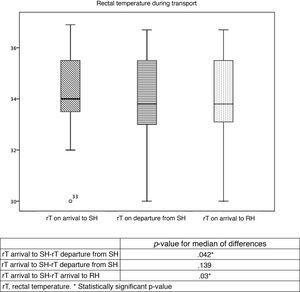
Table 2 and Fig. 2 summarise the temperatures recorded in the patients. Table 3 presents trends in rTs during stabilization and transport. Although the target temperature was not achieved in patients classified as warm, there was still a significant decrease in temperature between the arrival of the team to the SH and the handoff of the patient in the RH, although the differences in temperature between rTs during transport and on arrival to the RH were not significant. There were also no significant changes in the rT of patients in the overcooled or target range categories. We did not find differences in the proportion of overcooled patients based on the measures used to deliver nscTH. In 37 patients, we were able to assess differences in rT based on the applied cooling methods; turning off heat sources achieved a decrease in rT of 2.7 °C, and the external application of icepacks further decreased temperature by 1.7 °C. We also found no differences in temperature stability or the achievement of the target temperature and the duration of transport.
Stability of rectal temperature during transport.
| n | Mean rT | P | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SH arrival | SH departure | RH arrival | SH arrival | SH departure | RH arrival | SH arrival-SH departure | SH departure–RH arrival | SH arrival–RH arrival | |
| Overcooling (< 33 °C) | 2 | 8 | 7 | 31 | 31.2 | 31.2 | .5 | .18 | .5 |
| Target (33-34 °C) | 12 | 11 | 12 | 33.5 | 33.1 | 33.3 | .02* | .14 | .29 |
| Warm (> 34 °C) | 14 | 26 | 26 | 35.7 | 35.2 | 35 | .04* | .06 | .03* |
aT, axillary temperature; RH, receiving hospital; rT, rectal temperature; SH, sending hospital; tT, target temperature.
There were no differences in rT based on disease severity (intubation, cardiac massage, inotropic support) or the use of sedation.
DiscussionThis is the first prospective study in patients with HIE managed with nscTH during transport by a specialised paediatric and neonatal transport team in Spain. The observed perinatal events and patient characteristics were similar to those described in the previous literature.1,23
Although nscTH has proven effective and safe for use in transport24–27 and was initiated consistently at the SH, only one fourth of patients reached the RH with temperatures in the target range, a finding that was also consistent with the literature (25-30%).10,24,28 Some patients reached the target at some point, but did not arrive to the RH in the target range. It is important to consider that neonates with HIE are at high risk of overcooling due to their natural tendency to maintain a rT around 34.5 °C8,29,30 and that delivery of nscTH with icepacks has been associated with overcooling when central or rectal temperatures are not monitored continuously or measured every 15 to 20 minutes,25,31,32 and the percentage of overcooled patients in our sample was not as high as the percentages found in other studies (10-30%).25,26,28,33 The reason that we found no differences in the percentage of overcooled patients based on the methods used for cooling was probably the continuous monitoring of rT.
Achieving the target temperature in patients in the overcooling range with non-servocontrolled methods is challenging. In addition, avoiding hyperthermia is crucial, as each 1 °C increment in central temperature (cT) starting from 37 °C is associated with an up to 4-fold increase in the odds ratio for death or moderate to severe neurologic impairment.16,34 More than two thirds of patients arrived to the RH in the warm range, as described in previous studies.25,31 Nevertheless, there was a cumulative decrease of half a degree throughout the stabilization and transport stages compared to each of them in isolation, which may suggest that the cooling methods used in the study can achieve a decrease in cT if implemented from the outset at the SH and maintained throughout transport, although not quickly or thoroughly enough to achieve and maintain the target temperature within 30-60 minutes.24,31 The time to stabilization was a bit shorter compared to the times reported by other transport teams.24,35
In this case series, and in agreement with the previous literature on the subject, there were no deaths during transport, and the incidence of complications during the interhospital transport of neonates with HIE was low, suggesting that transport of these patients under nscTH, despite its complexity, is safe as long as it is performed by specialised teams and following evidence-based protocols.
We ought to highlight that the cT had only been monitored in half of the patients at the SH, which hindered our assessment of the impact of the measures used for cooling. Other authors have also described difficulties monitoring cT when the necessary resources are not available at the time of delivery of nscTH, and we found a considerable percentage of patients that were overcooled when the transport team arrived, probably due to the application of cooling measures without close monitoring of cT.1,30–32 One possible explanation would be patients needing airway management and establishment of central vascular access, which could delay the monitoring of temperature until the initial stabilization was complete before the departure to the RH. Paradoxically, our findings show that the delivery of nscTH is most effective during patient stabilization at the SH. In any case, the number of patients monitored by the transport team increased gradually through the study period. This evinces the importance of continuing education in specialised transport teams and their coordination with the referring intensive care units.1 We ought to highlight that both rectal and oesophageal temperatures are good indicators of cerebral cT, but neither axillary nor tympanic temperatures correlate to rT reliably in the context of hypothermia.6,32
Another of the limitations of our study was that 8 severely ill patients were lost to follow-up, highlighting the difficulty of stabilizing these patients and adding an additional task to it, as would be the management of nscTH. To this we must add the difficulty of performing neurologic evaluations in neonates for both staff at the SH and the transport team. Scales for assessment and grading of HIE offer a standardised, systematic and organised approach to estimate disease severity that can be used by professionals with varying degrees of training in the assessment of these patients. The complexity of assessing the neurologic status of a neonate, which has given rise to a subspeciality, if an unrecognised one, in most neonatal intensive care units, reflects the importance of providing support to teams that need to evaluate these patients.1,4,9,36 Thus, in the case that it is unclear whether HIE should be categorised as mild or moderate, TH should be initiated as soon as possible and the patient transported to a RH. There is no evidence on the beneficial or deleterious effects of lowering body temperature below normal or even to the hypothermic range in neonates with perinatal asphyxia without waiting to establish whether they have moderate or severe HIE,1,37 and transient changes in mental status have been described in infants with asphyxia, which hinders their evaluation at a specific time point without the use of other diagnostic tools, such as amplitude-integrated encephalography or head ultrasound.3,4,17–20
The most important finding of our study is that initiating nscTH with frequent monitoring of rT from arrival of the transport team to the SH achieved a significant decrease in rT that was not observed when these measures were implemented solely during transport. It is reasonable to hypothesise that if these measures were implemented in the initial management of the patient by the staff of the SH, the decrease could be even greater. However, although due to the geographical characteristics of our catchment area most neonates reach the RH by 6 hours post birth (which is not frequent in other transport teams31–33), only half of the infants arrived by 4 hours post birth. Data published by the Vermont Oxford Neonatal Encephalopathy Registry shows that more than 60% of neonates with HIE are born in facilities other referral hospitals experienced in TH,38 and there is a delay of up to 2 hours in the achievement of the target temperature in infants born outside RHs.3,12,24,27 Thus, there is inequality in the management of these neonates compared to those born in referral centres, as there is evidence that TH is more effective the quicker the target temperature is reached following the hypoxic-ischaemic insult.1,3,31,33,39
Multiple studies have demonstrated that servocontrolled TH (scTH) during neonatal transport is feasible, safe and more efficient than nscTH, decreasing the time from birth and initiation of cooling to reaching the target temperature, achieving more stable temperatures and decreasing the risk of overcooling.9,24,28,31–33,39 These studies also show that maintaining the target temperature is more difficult during transport compared to the intensive care unit setting.32,40 After the introduction of scTH in patient transport, published results on the time to stabilization have been heterogeneous, with some authors reporting longer times that could be attributed to a more meticulous preparation to ensure safe transport, while others report shorter times that could be due to the use of automated servocontrolled systems allowing the staff to focus fully on patient stabilization.24,28,32,40 Lastly, we ought to mention that weighing the cost of implementing scTH in transport against the estimated direct and indirect costs to the health system of children developing cerebral palsy suffices to justify the implementation of this treatment during transport.4
ConclusionPaediatric and neonatal transport teams offer adequate response times to patients with moderate-to-severe suspected HIE, and most patients reach the RH within 6 hours of birth. It is important to monitor cT from initiation of nscTH at the SH. There is clearly room to improve the efficacy of nscTH during transport. Servocontrolled scTH is an alternative that would allow a finer initial stabilization, as it offers improved temperature control and earlier achievement of the target temperature, thus making the neuroprotection “cold chain” more effective and extending the therapeutic options available in referral hospitals to infants born outside them.
Please cite this article as: Torre Monmany N, Maya Gallego S, Esclapés Giménez T, Sardà Sánchez M, Rodríguez Losada O, Martínez Planas A, et al. Retos en la aplicación de la hipotermia terapéutica no servo-controlada durante el transporte neonatal en Cataluña. An Pediatr. 2021;95:459–466.
The study was presented at the Virtual Congress of the European Academy of Paediatric Societies, October 2020.