Although malignant tumours are rare in the paediatric population, with an incidence of 15 new cases per 100 000 children under 14 years per year, they are the second leading cause of death overall and the leading cause of death due to disease in this age group. While diagnosis of a malignancy is rare in children, it is even less common in the first years of life. The current issue of Anales de Pediatría includes an interesting review of the cases of 72 patients aged less than 18 months with malignant tumours managed in a single Spanish hospital in the past 12 years that analysed the differential features of cancer at this young age.1
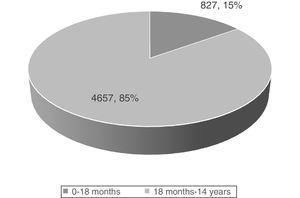
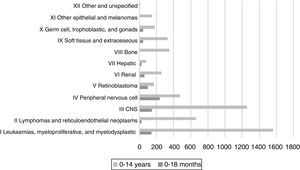
In agreement with the records of other childhood cancer registers in different countries, and based on data corresponding to the past 5 years in the Spanish Register of Childhood Tumours of the Sociedad Española de Hematología y Oncología Pediátricas (RETI-SEHOP), patients aged less than 18 months account for 15% of all malignant tumours diagnosed in children under 14 years (Fig. 1).2 While the most frequent malignant tumours in children are leukaemias (30%), central nervous system (CNS) tumours (20%), lymphomas (15%) and tumours of the neural crest (10%), in the group aged less than 18 months leukaemias are the third most frequent type of tumour, with a frequency below that of embryonal tumours, such as tumours of the neural crest (29%), CNS tumours (17.4%) or retinoblastoma (11%).2 In fact, up to 50% of neural crest tumour, nearly 60% of retinoblastomas, more than 30% of hepatoblastomas and more than 20% of childhood renal tumours are diagnosed in the first 18 months of life (Fig. 2).2
New cases registered in the RETI-SEHOP, 2015-2019 period.2
Between 5% and 10% of childhood cancers are hereditary. This hereditary nature is particularly relevant in the first years of life in patients with retinoblastoma, Wilms tumour or neuroectodermal syndromes (such as neurofibromatosis or tuberous sclerosis).
Early diagnosis allows detection of the disease in early stages, when the probability of cure is greater. Taking into account that the presenting symptoms of malignant tumours may overlap with those of other milder diseases in childhood, recognising warning symptoms and signs may be particularly challenging in the youngest patients. Thus, it is important to pay particular attention to signs and symptoms such as prolonged fever, faltering growth, irritability, pallor, ecchymosis, macrocephaly, leukocoria, abdominal distension, hepatomegaly, splenomegaly, focal neurologic signs, diarrhoea, constipation, persistent lymphadenopathy or skin lesions, among others. It is the severity of these manifestations, their persistence or progression or their poor outcome despite treatment that should be considered red flags. Leukocoria is clearly one of the few warning signs the presence of which warrants immediate referral for ophthalmological evaluation. For this reason, assessment of the pupillary light reflex at birth and in successive routine check-up visits in healthy children is essential for the early diagnosis of retinoblastoma, which would allow visual and ocular preservation.
As is the case of cancers in adults and older children, at present the treatment of malignant tumours in young children is based on 4 tools: surgery, chemotherapy, radiation and immunotherapy. The most suitable approach is determined based on location and cancer subtype. In recent years, most patients have been treated in the context of clinical trials or in adherence with established international guidelines. One of the biggest challenges in the treatment of the youngest children is determining the appropriate dosage of cytostatic agents. Despite the lack of data, empirical adjustment of the dosage is the norm in infants and young children based on observed improvements in the safety and tolerability of treatments. The impact of such heterogeneous dosage in infants and young children on the toxicity and efficacy of different cytostatic agents has not been studied in depth. Most protocols include dose adjustments of commonly used cytostatic agents based on age, weight or body surface area using mathematical formulas (for example, the 30 kg = 1 m2 rule), but once the patient exceeds the given threshold (such as age > 12 months), treatment continues with the standard dose based on body surface area, which entails a substantial increase in the administered dose. Pending the establishment of a more objective method for dosing, some groups are working on developing dosage tables based on body surface area to better fine-tune dose adjustments that take into account markers of clearance for different drugs.3
In very young children, supportive and holistic care delivered by a multidisciplinary team is necessary to minimise the incidence of acute and late complications associated with cancer, with particular emphasis on the maintenance of permanent vascular access lines, pain management and anaesthesia in diagnostic and therapeutic procedures, nutritional support, infection prevention and treatment and neurodevelopment.
In Spain, the current 5-year survival for childhood cancer overall is currently 80%,2 similar to survival in other developed countries, and in this context, the survival for some of the tumours diagnosed in the first years of life (such as retinoblastoma, nephroblastoma, localized neuroblastoma) exceeds 90%. Surgery, radiotherapy, chemotherapy, immunotherapy, recurrent use of anaesthesia for performance of diagnostic or therapeutic procedures and the acute complications of treatment may cause long-term sequelae, especially when used in the first years of life. For this reason, the goals of treatment no longer focus exclusively on pursuing cure, but also prioritize prevention, diagnosis and monitoring of long term adverse effects. To this end, it is particularly important to understand and predict how cancer and its treatment may affect neurodevelopment in infancy and early childhood and schedule periodic evaluations by specialists to follow-up these patients and deliver appropriate interventions through early intervention units.4 Meeting the psychosocial needs of young children with cancer poses a unique challenge, as these patients experience abrupt changes in many developmental milestones.4
Lastly, we must not forget the psychological impact that diagnosis of cancer in infants or young children has on their families (parents, siblings, grandparents, caregivers), and the importance of offering support and follow-up through mental health services.5
Cancer in the first 18 months of life is a challenge that demands interdisciplinary care offering patients not only the highest chances of cure and close monitoring of possible adverse events of treatment, but also facilitate the appropriate interventions to ensure the highest possible degree of social integration in subsequent stages of life.
Please cite this article as: Fernández-Teijeiro Álvarez A. Cáncer en los primeros 18 meses de vida. An Pediatr (Barc). 2020;93:355–357.